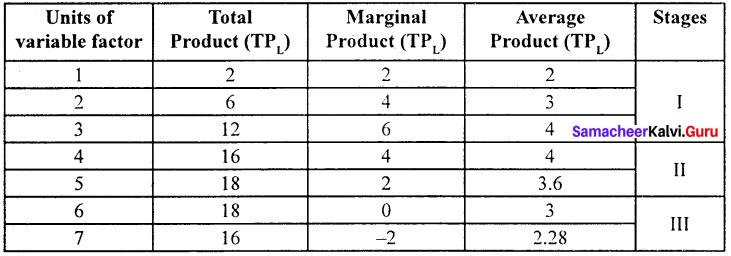
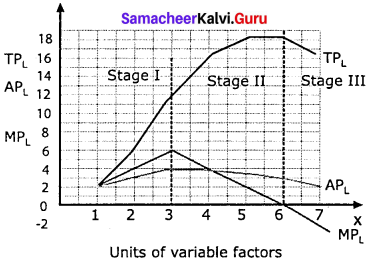
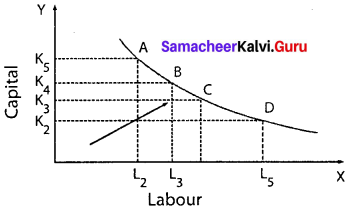
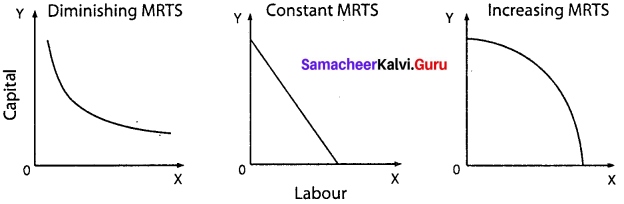
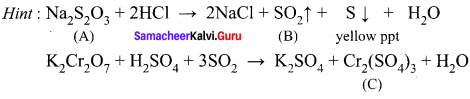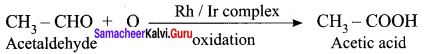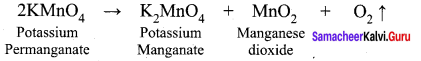Students can Download Maths Chapter 3 Algebra Ex 3.2 Questions and Answers, Notes Pdf, Samacheer Kalvi 7th Maths Book Solutions Guide Pdf helps you to revise the complete Tamilnadu State Board New Syllabus and score more marks in your examinations.
Tamilnadu Samacheer Kalvi 7th Maths Solutions Term 2 Chapter 3 Algebra Ex 3.2
Question 1.
Fill in the blanks.
(i) Unit digit of 124 × 36 × 980 is ______
(ii) When the unit digit of the base and its expanded form of that number is 9, then the exponent must be _______ power.
Answers:
(i) 0
(ii) Odd
Question 2.
Match the following:
Group A | Group B | ||
| (i) | 2010 | (a) | 6 |
| (ii) | 12111 | (b) | 4 |
| (iii) | 44441 | (c) | 0 |
| (iv) | 25100 | (d) | 1 |
| (v) | 71683 | (e) | 9 |
| (vi) | 729725 | (f) | 5 |
Answer:
(i) – c
(ii) – d
(iii) – b
(iv) – f
(v) – a
(vi) – e
Question 3.
Find the unit digit of expanded form.
(i) 2523
(ii) 1110
(iii) 4615
(iv) 10012
(v) 2921
(vi) 1912
(vii) 2425
(viii) 3416
Solution:
(i) 2523
Unit digit of base 25 is 5 and power is 23. Thus the unit digit of 2523 is 5.
(ii) 1110
Unit digit of base 11 is 1 and power is 10. Thus the unit digit of 1110 is 1.
(iii) 4615
Unit digit of base 46 is 6 and power is 15. Thus the unit digit of 4615 is 6.
(iv) 10012
Unit digit of base 100 is 0 and power is 12. Thus the unit digit of 10012 is 0.
(v) 2921
Unit digit of base 29 is 9 and power is 21 (odd power).
Therefore, unit digit of 2921 is 9.
(vi) 1912
Unit digit of base 19 is 9 and power is 12 (even power).
Therefore, unit digit of 1912 is 1.
(vii) 2425
Unit digit of base 24 is 4 and power is 25 (odd power).
Therefore, unit digit of 2425 is 4.
(viii) 3416
Unit digit of base 34 is 4 and power is 16 (even power).
Therefore, unit digit of 3416 is 6.
Question 4.
Find the unit digit of the following numeric expressions.
(i) 11420 + 11521 + 11622
(ii) 1000010000 + 1111111111
Solution:
(i) 11420 + 11521 + 11622
In 11420 unit digit of base 114 is 4 and power is 20 (even power).
∴ Unit digit of 11420 is 6.
In 11521 unit digit of base 115 is 5 and power is 21 (Positive Integer).
∴ Unit digit of 11521 is 5.
In 11622 unit digit of base 116 is 6 and power is 22 (Positive Integer).
∴ Unit digit of 11622 is 6.
∴ Unit digit of 11420 + 11521 + 11622 can be obtained by adding 6 + 5 + 6 = 17.
Unit digit of 11420 + 11521 + 11622 is 7.
(ii) 1000010000 + 1111111111
In 1000010000 the unit digit of base 10000 is 0 and power is 10000.
Unit digit of 1000010000 is 0.
In 1111111111 the unit digit of base 11111 is 1 and power is 11111.
Unit digit of 1111111111 is 1.
Unit digit of 10000100000 + 1111111111 is 0 + 1 = 1
Objective Type Question
Question 5.
Observe the equation (10 + y)4 = 50625 and find the value of y.
(i) 1
(ii) 5
(iii) 4
(iv) 0
Answer:
(ii) 5
Question 6.
The unit digit of (32 × 65)0 is
(i) 2
(ii) 5
(iii) 0
(iv) 1
Answer:
(iv) 1
Question 7.
The unit digit of the numeric expression 1071 + 1072 + 1073 is
(i) 0
(ii) 3
(iii) 1
(iv) 2
Answer:
(i) 0
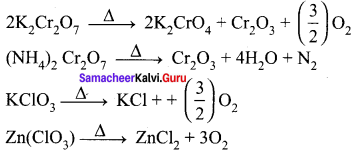
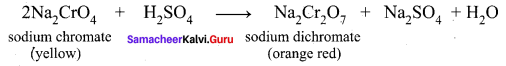
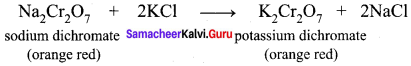
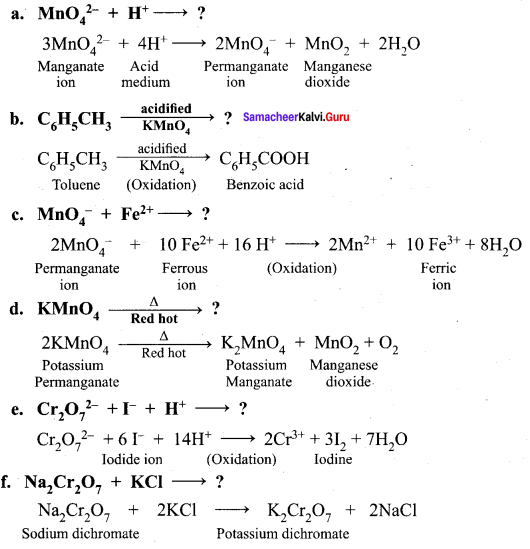
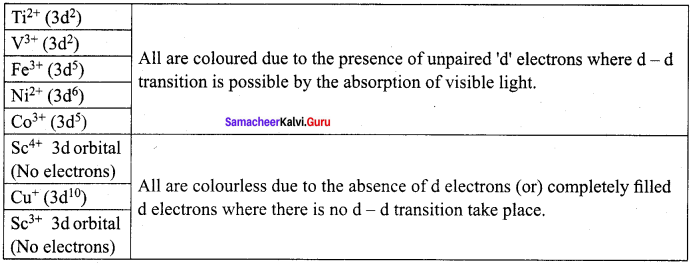
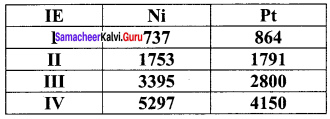
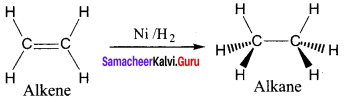
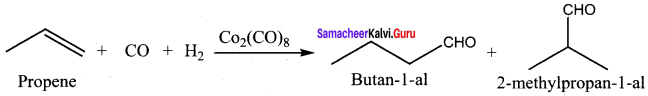
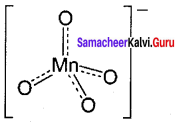
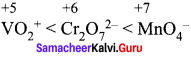 greater the oxidation state, higher is the oxidising power.
greater the oxidation state, higher is the oxidising power.