You can Download Samacheer Kalvi 9th Science Book Solutions Guide Pdf, Tamilnadu State Board help you to revise the complete Syllabus and score more marks in your examinations.
Tamilnadu Samacheer Kalvi 9th Science Solutions Chapter 15 Carbon and its Compounds
Samacheer Kalvi 9th Science Carbon and its Compounds Textbook Exercises
I. Choose the correct answer.
Chapter 15 Carbon And Its Compounds Question 1.
A phenomenon in which an element exists in different modification in same physical state is called ……………….
(a) isomerism
(b) allotropy
(c) catenation
(d) crystallinity
Answer:
(b) allotropy
Carbon And Its Compounds 9th Class Question 2.
Number of free electron(s) in each carbon of graphite is ………………….
(a) one
(b) two
(c) three
(d) four
Answer:
(c) three
Carbon And Its Compounds Class 9 Samacheer Question 3.
Carbon forms large number of organic compounds due to ………………………
(a) Allotropy
(b) Isomerism
(c) Tetravalency
(d) Catenation
Answer:
(a) Allotropy
9th Science Carbon And Its Compounds Question 4.
Which of the following does not contain double bond?
(a) CO2
(b) C2H4
(C) HCl
(d) O2
Answer:
(C) HCl
Carbon And Its Compounds Class 9 Question 5.
Raagav brings his lunch every day to school in a plastic container which has resin code number 5. The container is made of …………….
(a) Polystyrene
(b) PVC
(c) Polypropylene
(d) LDPE
Answer:
(c) Polypropylene
Carbon And Its Compounds Samacheer Kalvi Question 6.
Plastics made of Polycarbonate (PC) and Acrylonitrile Butadiene Styrene (ABS) are made of resin code ………………
(a) 2
(b) 5
(c) 6
(d) 7
Answer:
(d) 7
Carbon And Its Compounds Question 7.
The lead pencil contains ………………
(a) graphite
(b) diamond
(c) lead
(d) charcoal
Answer:
(a) Graphite
Carbon And Its Compounds Solutions Question 8.
Graphene is one atom thick layer of carbon obtained from ……………..
(a) diamond
(b) fullerene
(c) graphite
(d) gas carbon
Answer:
(c) graphite
Class 9 Science Chapter 15 Notes Question 9.
The legal measures to prevent plastic pollution come under the ……………….. Protection Act 1988.
(a) forest
(b) wildlife
(c) environment
(d) human rights
Answer:
(c) Environment
II. Fill in the blanks.
- ………….. named carbon.
- Buckminster Fullerene contains ……………. carbon atoms.
- Compounds with same molecular formula and different structural formula are known as ………………
- Different methods of formation of carbon is the main reason for its ………………..
- There are …………….. plastic resin codes.
Answer:
- Antoine Lavoisier
- 60
- isomer
- Allotropy
- 7
III. Match the following.
| S.No. | A | B |
| 1. | Alkyne | (a) Bucky Ball |
| 2. | Andre Geim | (b) Oxidation |
| 3. | C60 | (c) Graphene |
| 4. | Thermocol | (d) Triple bond |
| 5. | Burning | (e) Polystyrene |
Answer:
- (d) Triple bond
- (c) Graphene
- (a) Bucky Ball
- (e) Polystyrene
- (b) Oxidation
IV. Answer in brief.
Class 9 Science Chapter 15 Exercise Questions And Answers Question 1.
Differentiate graphite and diamond.
Answer:
| Graphite | Diamond |
| Each carbon has three covalent bonds. | Each carbon has four covalent bonds |
| Soft, slippery to touch and opaque. | Hard, heavy and transparent. |
| It has tetrahedral units linked in three dimensions. | It has planar layers of hexagon units. |
| It is a non-conductor of heat and electricity. | It is conductor of heat and electricity. |
Science Chapter 15 Class 9 Question 2.
What are saturated and unsaturated compounds?
Answer:
Saturated carbon compounds are called alkanes as they have single bond between carbon atoms. Unsaturated carbon compounds are called alkenes as they have one or more double bonds between carbon atoms.
Question 3.
Carbon do not form ionic compounds. Why?
Answer:
Carbon shares electrons only through covalent bonding, hence it does not form ionic compounds.
Question 4.
What is the valency of carbon in carbon monoxide?
Answer:
Valency of carbon in carbon monoxide is 2.
Question 5.
Why are one-time use and throwaway plastics harmful?
Answer:
Use and throwaway plastics cause short and long-term environmental damage as they are difficult to recycle. They stay in an environment for over 1000 years.
These block drains and pollute water bodies. One-time use plastic causes health problems for humans, plants and animals. Some examples are plastic carry bags, cups, etc.
V. Answer in detail.
Question 1.
What is catenation? How does carbon form catenated compounds?
Answer:
Catenation is the binding of an element to itself or with other elements through covalent bonds to form open chain or closed chain compounds. .
Carbon is the most common element which undergoes catenation and forms long chain compounds. Carbon atom links repeatedly to itself through covalent bond to form linear chain, branched chain or ring structure. This property of carbon itself is the reason for the presence of large number of organic carbon compounds. So organic chemistry essentially deals with catenated carbon compounds.
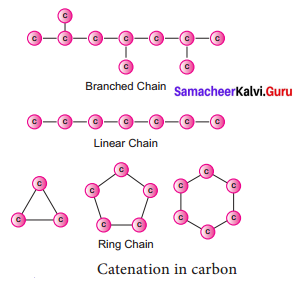
Question 2.
What are the chemical reactions of carbon?
Answer:
Elemental carbon undergoes no reaction at room temperature and limited number of reactions at elevated temperatures. But its compounds undergo large number of reactions even at room temperature.
Oxidation – (Reaction with oxygen)
Carbon combines with oxygen to form its oxides like carbon monoxide (CO) and carbon dioxide (CO2) with evolution of heat. Organic carbon compounds like hydrocarbon also undergo oxidation to form oxides and steam with evolution of heat and flame. This is otherwise called Burning,
2C(s) + O2(g) ➝ 2CO(g) + heat
C(s) + O2(g) ➝ CO2(g) + heat
- Reaction with steam
Carbon reacts with steam to form carbon monoxide and hydrogen. This mixture is called water gas.
C(s) + H(s)O(g) ➝ CO(g) + H2(g) - Reaction with sulphur
With sulphur, carbon forms its disulphide at high temperature.
C(s) + S(s) ➝ CS2(g) - Reaction with metals
At elevated temperatures, carbon reacts with some metals like iron, tungsten, Titanium, etc. to form their carbides.
W(s) + C(g) ➝ WC(s)
Question 3.
Name the three safer resin codes of plastics and describe their features.
Answer:
| Resin code 2 (PEHD) | It is light, strong and can be recycled |
| Resin code 4 (PELD, LLDPE) | It is very flexible, soft but strong |
| Resin code 5 (PP) | It feels waxy. It is light and hard but scratches easily. |
VI. HOTS
Question 1.
Why do carbon exist mostly in combined state?
Answer:
Carbon is an element that can form many different compounds, as each carbon atom can form 4 chemical bonds with other atoms and because the carbon atom is just the right size to fit in comfortably as parts of very large molecules.
Question 2.
When a carbon fuel burns in less aerated room, it is dangerous to stay there. Why?
Answer:
When carbon fuel bums in less aerated room, carbon monoxide is formed. When people are exposed to CO, it enters into human body through breathing and affects the function of haemoglobin. CO displaces oxygen from haemoglobin thereby stops its function (supply of oxygen to the parts of body) leading to death.
Question 3.
Explain how dioxins are formed? Which plastic type they are linked to and why they are harmful to humans?
Answer:
Burning PVC plastic releases dioxins which are one of the most dangerous chemicals known to humans.
Question 4.
Yugaa wants to bdy a plastic water bottle. She goes to the shop and sees four different kinds of plastic bottles with resin codes 1, 3, 5 and 7. Which one should she buy? Why?
Answer:
Yuga should by plastic bottle with resin code 5, as it is considered to be more safer.
Activity
Question 1.
With the help of your teacher, try to classify the following compounds and materials and, . fill in the table accordingly. HCN, CO2, Propane, PVC, CO, Kerosene, LPG, Coconut oil, Wood, Perfume, Alcohol, Na2CO3, CaCO3, MgO, Cotton, Petrol.
Answer:
| Inorganic | Organic |
| MgO | PVC |
| CO2 | Alcohol |
| CO | Propane |
| CaCO3 | HCN |
| Na2CO3 | Kerosene |
| LPG | |
| Petrol | |
| Coconut oil | |
| Perfume | |
| Wood | |
| Cotton |
Samacheer Kalvi 9th Science Carbon and its Compounds Additional Questions
I. Answer briefly.
Question 1.
Explain carbon cycle or biogeochemical cycle.
Answer:
The carbon cycle is a biogeochemical cycle by which carbon is exchanged among the biosphere, geosphere, hydrosphere and atmosphere of the Earth. Carbon is the main component of biological compounds as well as major component of many minerals such as lime stone.
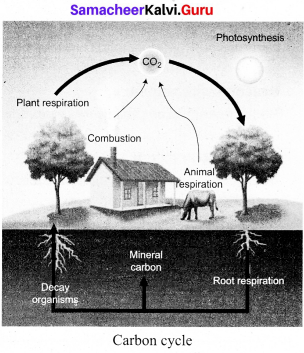
Steps in carbon cycle:
- Carbon enters the atmosphere as CO2 from respiration and combustion.
- CO2 is absorbed by producers to make carbohydrates in photosynthesis.
- Animals feed on the plant passing the carbon compounds along the food chain. Most of the carbon they consume is exhaled as CO2, formed during respiration. The animals and plants eventually die.
- The dead organisms are eaten by decomposers and the carbon in their bodies is returned to the atmosphere as CO2. The plant and animal material may then be available as fossil fuel in the future for combustion.
Question 2.
What is isomerism? Explain with an example.
Answer:
Isomerism is a special feature of catenated organic compounds.
For example, if we consider the molecular formula of an organic compound to be C2H6O, we will not be able to name the compound. This is because the molecular formula of an organic compound represents only the number of different atoms present in that compound. It does not indicate the way in which the atoms are arranged and hence its structure.
The phenomenon in which the same molecular formula may exhibit different structural arrangements is called isomerism.
The given formula C2H6O can be represented with two kinds of arrangements.
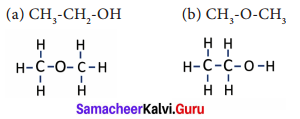
Both the compounds have same molecular but different kind of arrangement. In compound ‘a’ the oxygen atom is attached to a hydrogen and a carbon. It is an alcohol. Whereas, compound ‘b’ the oxygen atom is attached to two carbon atoms and it is an ether. Both these compounds have different physical and chemical properties.
Question 3.
Write a short note on graphene.
Answer:
Graphene is most recently produced allotrope of carbon which consists of honeycomb-shaped hexagonal ring repeatedly arranged in a plane. Graphene is the thinnest compound known to man at one atom thick. It is the lightest material known (with 1 square metre weighing around 0.77 milligrams) and the strongest compound discovered (100-300 times stronger than steel).
It is the best conductor of heat at room temperature. Layers of graphene are stacked on top of each other form graphite, with an interplanar spacing of 0.335 nanometres. The separate layers of graphene in graphite are held together by Vander Waals forces.
Question 4.
What is carbon monoxide? Why is it toxic?
Answer:
Carbon monoxide is a toxic gas of carbon. When fuels undergo incomplete combustion, it results in the formation of carbon monoxide. It is released into the atmosphere from various sources like vehicle fuels, domestic fuels, industries, furnaces, etc, cigarette smoking also is a source of carbon monoxide.
Carbon monoxide is an odorless toxic gas. When CO enters the human body through breathing, it affects the function of hemoglobin. CO displaces oxygen from hemoglobin thereby stopping its function, i.e., a supply of oxygen is blocked to the parts of the body, thereby leading to death.
Question 5.
What are the drawbacks of plastics?
Answer:
Drawbacks of plastics
- Plastics take a long time to fully break down in nature.
- The microbes that breakdown plastics are too few and the quantity of plastics we produce is too many.
- A lot of plastics does not get recycled and ends up polluting the environment.
- Some plastics contain harmful chemical additives that are not good for human health.
- Burning of plastics releases toxic gases that are harmful to health and environment
- One time use and throwaway plastics end up littering and polluting the environment.
Question 6.
What is a resin code? What is the need to display them on plastic articles?
Answer:
The resin code is a set of symbols appearing on plastic products that is helpful in the identification of the type of polymer used to make that product.
Platic needs to be recycled or disposed of safely. Each plastic is composed of a different polymer or set of molecules. Different molecules do not mix when plastics are recycled. For this reason, plastics need to be segregated before recycling. These resin codes help in easy segregation of plastic products to be recycled.
Question 7.
A survey showed that one time use plastic items were among the top 10 plastic items found in garbage washed up from oceans. Can this be true? Explain how.
Answer:
Use and throwaway plastics cause short and long time environmental damage. These items like plastic carry bags, straws, plates, spoons, pouches, etc., take a few seconds to be made in a factory, but can stay in the environment forever a 1000 years. These items block drains and pollute water bodies. These plastics break down into pieces that are smaller than 5mm in diameter. These microplastics pollute the ocean and harm the marine life, who mistake them as their food.
These one time use plastics when disposed carelessly are carried to the ocean by winds, cause harm to marine life and are brought back to the shore by waves.