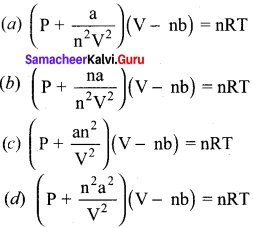
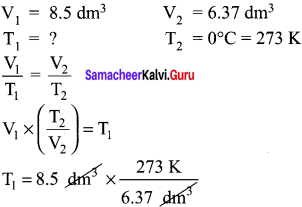
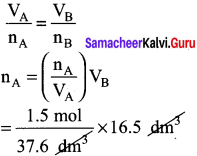
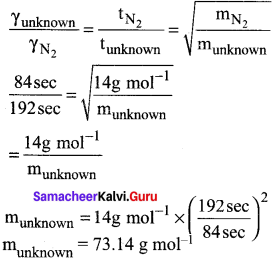
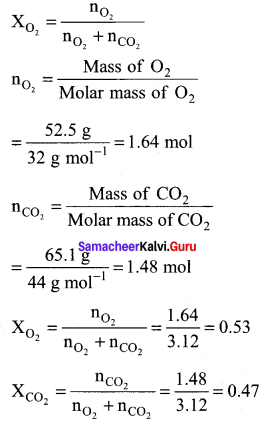
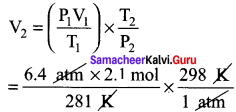
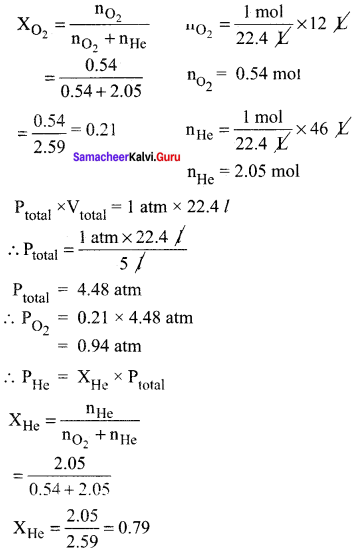
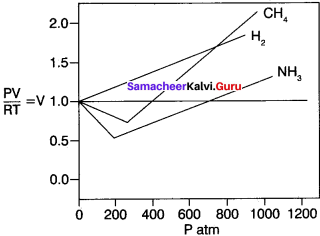
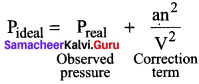
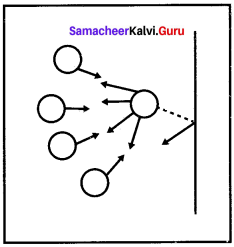
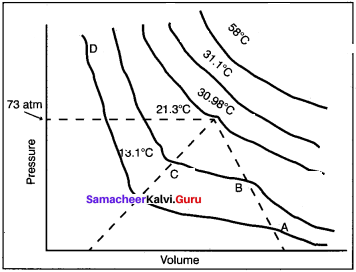
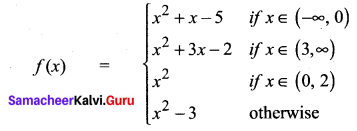
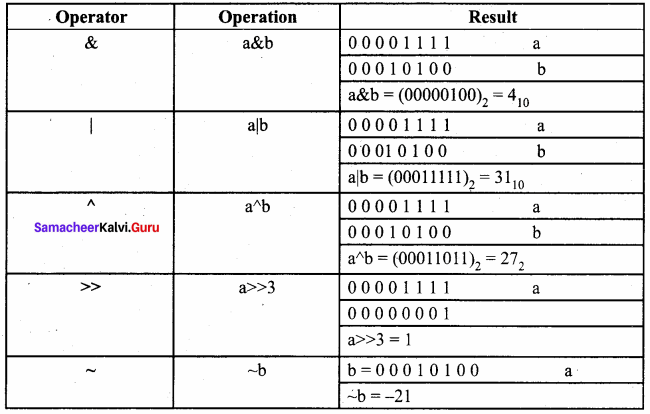
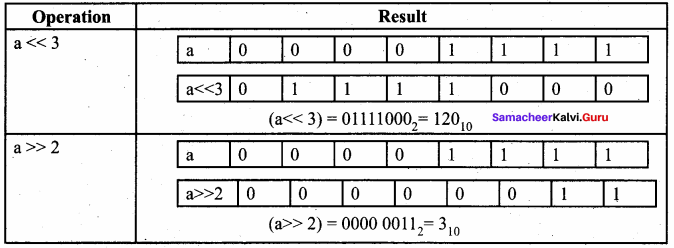
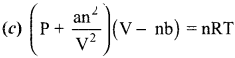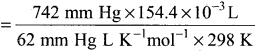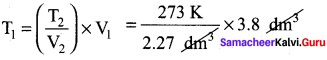Students can Download Accountancy Chapter 1 Introduction to Accounting Questions and Answers, Notes Pdf, Samacheer Kalvi 11th Accountancy Book Solutions Guide Pdf helps you to revise the complete Tamilnadu State Board New Syllabus and score more marks in your examinations.
Tamilnadu Samacheer Kalvi 11th Accountancy Solutions Chapter 1 Introduction to Accounting
Samacheer Kalvi 11th Accountancy Introduction to Accounting Text Book Back Questions and Answers
I. Multiple Choice Questions
Choose the Correct Answer
Samacheer Kalvi Guru 11th Accountancy Question 1.
The root of financial accounting system is ……………..
(a) Social accounting
(b) Stewardship accounting
(c) Management accounting
(d) Responsibility accounting
Answer:
(b) Stewardship accounting
11th Accountancy Chapter 1 Question 2.
Which one of the following is not a main objective of accounting?
(a) Systematic recording of transactions
(b) Ascertainment of the profitability of the business
(c) Ascertainment of the financial position of the business
(d) Solving tax disputes with tax authorities
Answer:
(d) Solving tax disputes with tax authorities
11th Accountancy 1st Chapter Question Answer Question 3.
Which one of the following is “hot a branch of accounting?
(a) Financial accounting
(b) Management accounting
(c) Human resources accounting
(d) None of the above
Answer:
(d) None of the above
Samacheer Kalvi 11th Accountancy Guide Question 4.
Financial position of a business is ascertained on the basis of ……………..
(a) Journal
(b) Trial balance
(c) Balance Sheet
(d) Ledger
Answer:
(c) Balance Sheet
Samacheer Kalvi Accountancy 11th Question 5.
Who is considered to be the internal user of the financial information?
(a) Creditor
(b) Employee
(c) Customer
(d) Government
Answer:
(b) Employee
II. Very Short Answer Questions
11th Accountancy Samacheer Kalvi Question 1.
Define accounting.
Answer:
American Accounting Association has defined accounting as “the process of identifying, measuring and communicating economic information to permit informed judgements and decisions by users of the information.”
Samacheer Kalvi 11th Accountancy Book Question 2.
List any two functions of accounting.
Answer:
The main functions of accounting:
- Measurement
- Forecasting
1. Measurement: The main function of accounting is to keep systematic record of transactions, post them in the ledger and ultimately prepare the final accounts.
2. Forecasting: With the help of the various tools of accounting, future performance and financial position of the business enterprises can be forecasted.
Accountancy Class 11 Pdf Samacheer Kalvi Question 3.
What are the steps involved in the process of accounting?
Answer:
Accounting is the systematic process of identifying, measuring, recording, classifying, summarising, interpreting and communicating financial information.
11th Accountancy 1st Chapter Question 4.
Who are the parties interested in accounting information?
Answer:
- Internal users: Owners, Management and Employees.
- External users: Creditors, Investors, Customers, Tax authorities, Government, Researchers and General Public.
Samacheer Kalvi 11th Accountancy Question 5.
Name any two basis of recording accounting information.
Answer:
There are three basis of accounting in common usage.
- Cash basis
- Accrual or mercantile basis
III. Short Answer Questions
Samacheer Kalvi 11th Accountancy Book Back Answers Question 1.
Explain the meaning of accounting.
Answer:
Accounting is the systematic process of identifying, measuring, recording, classifying, summarising, interpreting and communicating financial information. Accounting gives information on:
- The resources available
- How the available resources have been employed and
- The results achieved by their use.
11th Accountancy Solutions Samacheer Kalvi Question 2.
Discuss briefly the branches of accounting.
Answer:
The main branches of accounting are:
1. Financial Accounting: It involves recording of financial transactions and events.
2. Cost Accounting: It involves the collection, recording, classification and appropriate allocation of expenditure for the determination of the costs of products or services and for the presentation of data for the purpose of cost control and managerial decision making.
3. Management Accounting: It is concerned with the presentation of accounting information in such a way as to assist management in decision making and in the day – to – day operations of an enterprise.
4. Social Responsibility Accounting: It is concerned with presentation of accounting information by business entities and other organisations from the view point of the society by showing the social costs incurred such as environmental pollution by the enterprise and social benefits such as infrastructure development and employment opportunities created by them.
5. It is concerned with identification, quantification and reporting of investments made in human resources of an enterprise.
11th Accountancy Book Samacheer Kalvi Question 3.
Discuss in detail the importance of accounting.
Answer:
The importance of accounting is:
1. Systematic records: All the transactions of an enterprise which are financial in nature are recorded in a systematic way in the books of accounts.
2. Preparation of financial statements: Results of business operations and the financial position of the concern can be ascertained from accounting periodically through the preparation of financial statements.
3. Assessment of progress: Analysis and interpretation of financial data can be done to assess the progress made in different areas and to identify the areas of weaknesses.
4. Aid to decision making: Management of a firm has to make routine and strategic decisions while discharging its functions.
5. Satisfies legal requirements: Various legal requirements like maintenance of provident fund (PF) for employees, Tax deducted at source (TOS), filing of tax returns and properly fulfilled with the help of accounting.
6. Information to interested groups: Accounting supplies appropriate information to different interested groups like owners, management, creditors, employees, financial institutions, tax authorities and the Government.
7. Legal evidence: Accounting records are generally accepted as evidence in courts of law and other legal authorities in the settlement of disputes.
8. Computation of tax: Accounting records are the basic source for computation and settlement of income tax and other taxes.
9. Settlement during mergers: When two or more business units decide to merge, accounting records provide information for deciding the terms of merger and any compensation payable as a consequence of merges.
Account 11th 1st Chapter Question 4.
Why are the following parties interested in accounting information?
- Investors
- Government
Answer:
1. Investors: Persons who are interested in investing their funds in an organisation should know about the financial condition of a business unit while making their investment decisions. They are more concerned about future earnings and risk bearing capacity of the organisation which will affect the return to the investors.
2. Government: The scarce resources of the country are used by business enterprises. Information about performance of business units in different industries helps the government in policy formation for development of trade and industry, allocation of scarce resources, grant of subsidy, etc.
11th Accounts Samacheer Kalvi Solutions Question 5.
Discuss the role of an accountant in the modem business world.
Answer:
The important role of an accountant is:
1. Record keeper: The accountant maintains a systematic record of financial transactions.
2. Provider of information to the management : The accountant assists the management by providing financial information required for decision making and for exercising controls.
3. Protector of business assets: The accountant maintains records of assets owned by the business which enables the management to protect and exercise control over these assets.
4. Financial advisor: The accountant analysis financial information and advises the business managers regarding investment opportunities, strategies for cost savings, capital budgeting, provision for future growth and development, expansions of enterprise, etc.
5. Tax managers: The accountant ensures that tax returns are prepared and filed correctly on time and payment of tax is made on time
6. Public relation officer: The accountant provides accounting informations to various interest users for analysis as per their requirements.
Textbook Case Study Solved
Samacheer Kalvi 11th Accountancy Solutions Question 1.
How do SHGs maintain their accounting?
Answer:
They are maintaining single entry systems, so the cash book and personal accounts are being maintained by them. The nominal accounts are not being maintained by them, so we can not find out the accurate profit or loss on the business. We can find out the estimated value only.
11th Accountancy Guide Samacheer Kalvi Question 2.
Do you think that financial accounting system is suitable for all businesses?
Answer:
This system is not suitable for all business because the actual profit or loss cannot be found out by this system. If it is a small business only then we can follow this system.
Samacheer Kalvi 11th Accountancy Introduction to Accounting Additional Questions and Answers
I. Multiple Choice Questions
Choose the correct answer
11th Accounts Chapter 1 Question 1.
In 1494, ……………. an Italian developed double – entry book – keeping system.
(a) Luca Pacioli
(b) Kautilya
(c) Wheeler
(d) R.N.Cart
Answer:
(a) Luca Pacioli
Accountancy Class 11 Samacheer Kalvi Question 2.
Who is considered to be the external user of the financial information?
(a) Employee
(b) Owners
(c) Management
(d) Creditor
Answer:
(d) Creditors
11th Accountancy Chapter 1 Book Back Answers Question 3.
Which one is not a role of an accountant?
(a) Record keeper
(b) Tax manager
(c) PRO
(d) Owner
Answer:
(d) Owner
11th Accounts Book Samacheer Kalvi Question 4.
The first step of accounting cycle.
(a) Transactions
(b) Journlising
(c) Profit & loss account
(d) Trading account
Answer:
(a) Transactions
Samacheer Kalvi 11th Accountancy Book Pdf Question 5.
Original entry is otherwise called ………………
(a) Journal
(b) Ledger
(c) Trial balance
(d) Balance sheet
Answer:
(a) Journal
Accountancy 1st Chapter Question 6.
……………… is the language of business.
(a) Accounting
(b) Book – keeping
(c) Trade
(d) Banking
Answer:
(a) Accounting
Accountancy Book Class 11 Samacheer Kalvi Question 7.
Financial information for managerial decision making caused emergence of ……………… accounting.
(a) Management
(b) Cost
(c) Financial
(d) Corporate
Answer:
(a) Management
Samacheer Kalvi 11th Accounts Question 8.
Transferring the entries from the journal to the ledger ……………….
(a) Posting
(b) Journal
(c) Ledger
(d) Transaction
Answer:
(a) Posting
Question 9.
The balance in the trading account is the gross profit or ……………….
(a) Net profit
(b) Net loss
(c) gross loss
(d) balance
Answer:
(c) gross loss
Question 10.
A statement showing the balances of assets and liabilities is called as ……………….
(a) Profit & loss A/c
(b) Trading A/c
(c) Balance sheet
(d) Final A/c
Answer:
(c) Balance sheet
Question 11.
Two or more business units forming a single entity is known as ……………….
(a) Joint
(b) Merger
(c) Link
(d) Compound
Answer:
(b) Merger
Question 12.
………………. is irrecoverable debt ……………….
(a) debtor
(b) creditor
(c) bad debt
(d) loan
Answer:
(c) bad debt
Question 13.
Unsold goods lying in a business on a particular date are known as ……………….
(a) Stock
(b) creditor
(c) debtor
(d) cash
Answer:
(a) Stock
Question 14.
………………. is the incapability of a person or an enterprises to pay the debts
(a) Asset
(b) Liability
(c) Insolvency
(d) Sales
Answer:
(c) Insolvency
Question 15.
………………. is the amount incurred in order to produce and sell the goods and services.
(a) Creditor
(b) Debtor
(c) Stock
(d) Expense
Answer:
(d) Expense
II. Very Short Answer Questions
Question 1.
Write any two objectives of Accounting.
Answer:
- To keep a systematic record of financial transactions and events.
- To ascertain the profit or loss of the business enterprise.
Question 2.
Who are researchers?
Answer:
Researchers who carry out their research can use accounting information and make use of the published financial statements for analysis and evaluation.
Question 3.
Who is Public Relation Officer? (PRO)
Answer:
The accountant provides accounting information to various interested users for analysis as per their requirements.
Question 4.
What do you mean by legal evidence?
Answer:
Accounting records are generally accepted as evidence in courts of law and other legal authorities in the settlement of disputes.
Question 5.
Who is a creditor of business?
Answer:
A person who gives a benefit without receiving money or money’s worth immediately but to claim in future.
Question 6.
What is a bad debt?
Answer:
It is a loss to the business arising out of failure of a debtor to pay the dues. It is irrecoverable debt.
Question 7.
What is capital?
Answer:
It is the amount invested by the owner or proprietor in an organisation.
Question 8.
What is drawings?
Answer:
It is the amount of cash or value of goods, assets, etc., withdrawn from the business by the owner for the personal use of the owner.
Question 9.
What is a voucher?
Answer:
Any written or printed document in support of a business transaction is called a voucher.
Question 10.
What is depreciation?
Answer:
It refers to the gradual reduction in the value of fixed assets due to usage and passage of time.
III. Short Answer Questions
Question 1.
Write a note on
- Debtor
- Creditor
Answer:
- Debtor: A person who receives a benefit without giving money or money’s worth immediately, but liable to pay in future or in due course of time.
- Creditor: A person who gives a benefit without receiving money or money’s worth immediately but to claim in future.
Question 2.
Write a note on
- Purchases
- Sales.
Answer:
- Purchases: Buying of goods with the intention of resale is called purchase.
- Sales: When goods meant for resale are sold, it is called sales.
Question 3.
What is the difference between cash transaction and credit transactions?
Answer:
Cash transaction:
It is a transaction which involves immediate cash receipt or immediate cash payment.
Credit transaction:
It is a transaction in which cash is not received or paid immediately, but will be received or paid later.
Question 4.
What is the difference between voucher and invoice?
Answer:
Voucher:
Any written or printed document in support of business transaction is called a voucher. Examples: cash receipts, bank pay – in – slip, etc.
Invoice:
It is a statement prepared by a seller of goods to be sent to the buyer. It shows details of quantity, price, value, etc., of the goods and any discount given, finally showing the net amount payable by the buyer.