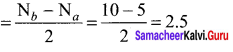Students who are in search of 11th Bio Botany exam can download this Tamilnadu State Board Solutions for 11th Bio Botany Chapter 15 Plant Growth and Development from here for free of cost. These cover all Chapter 15 Plant Growth and Development Questions and Answers, PDF, Notes, Summary. Download the Samacheer Kalvi 11th Biology Book Solutions Questions and Answers by accessing the links provided here and ace up your preparation.
Tamilnadu Samacheer Kalvi 11th Bio Botany Solutions Chapter 15 Plant Growth and Development
Kickstart your preparation by using this Tamilnadu State Board Solutions for 11th Bio Botany Chapter 15 Plant Growth and Development Questions and Answers get the max score in the exams. You can cover all the topics of Chapter 15 Plant Growth and Development Questions and Answers easily after studying the Tamilnadu State Board 11th Bio Botany Textbook solutions pdf.
Samacheer Kalvi 11th Bio Botany Plant Growth and Development Text Book Back Questions and Answers
Question 1.
Select the wrong statement from the following:
(a) Formative phase of the cells retain the capability of cell division.
(b) In elongation phase development of central vacuole takes place.
(c) In maturation phase thickening and differentiation takes place.
(d) In maturation phase, the cells grow further.
Answer:
(d) In maturation phase, the cells grow further.
Question 2.
If the diameter of the pulley is 6 inches, length of pointer is 10 inches and distance travelled by pointer is 5 inches. Calculate the actual growth in length of plant:
(a) 3 inches
(b) 6 inches
(c) 12 inches
(d) 30 inches
Answer:
(a) 3 inches
Question 3.
In uni sexual plants, sex can be changed by the application of:
(a) ethanol
(b) cytokinins
(c) ABA
(d) auxin
Answer:
(c) ABA
Question 4.
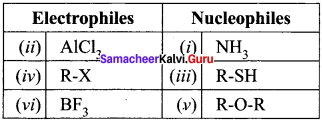
Select the correctly matched one:
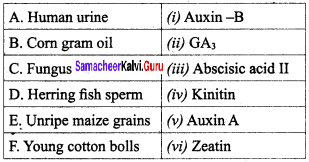
(a) A – (iii), B – (iv), C – (v), D – (vi), E – (i), F – (ii)
(b) A – (v), C – (ii), D – (iv), E – (vi), F – (iii)
(c) A – (iii), B – (v), C – (vi),D – (i), E – (ii), F – (iv)
(d) A – (ii), B – (iii), C – (v), D – (vi), E – (iv), F – (i)
Answer:
(b) A – (v), C – (ii), D – (iv), E – (vi), F – (iii)
Question 5.
Seed dormancy allows the plants to:
(a) overcome un favorable climatic conditions
(b) develop healthy seeds
(c) reduce viability
(d) prevent deterioration of seeds
Answer:
(a) overcome unfavorable climatic conditions
Question 6.
What are the parameters used to measure growth of plants?
Answer:
- Increase in length or girth (roots and stems)
- Increase in fresh or dry weight
- Increase in area or volume (fruits and leaves)
- Increase in number of cells produced.
Question 7.
What is plasticity?
Answer:
Plants follow different pathways in response to environment or phases of life to form different kinds of structures. This ability is called plasticity,
eg : Heterophylly in cotton and coriander. In such plants, the leaves of the juvenile plant are different in shape from those in mature plants.
On the other hand, the difference in shapes of leaves produced in air and those produced in water in buttercup also represent he heterophyllous development due to the environment. This phenomenon of heterophylly is an example of plasticity.
Question 8.
Write the physiological effects of Cytokinins.
Answer:
- Cytokinin promotes cell division in the presence of auxin (IAA).
- Induces cell enlargement associated with IAA and gibberellins
- Cytokinin can break the dormancy of certain light-sensitive seeds like tobacco and induces seed germination.
- Cytokinin promotes the growth of lateral bud in the presence of apical bud.
- Application of cytokinin delays the process of aging by nutrient mobilization. It is known as Richmond Lang effect.
- Cytokinin:
- increases rate protein synthesis
- induces the formation of inter-fascicular cambium
- overcomes apical dominance
- induces formation of new leaves, chloroplast and lateral shoots.
- Plants accumulate solutes very actively with the help of cytokinins.
Question 9.
Describe the mechanism of photoperiodic induction of flowering.
Answer:
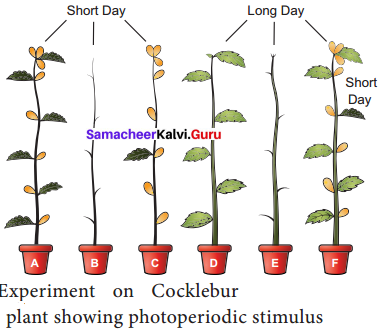
Photoperiodic stimulus is perceived by the leaves. Floral hormone is synthesised in leaves and translocated to the apical tip to promote flowering. This can be explained by a simple experiment on Cocklebur (Xanthium pensylvanicum), a short day plant. Usually Xanthium will flower under short day conditions. If the plant is defoliated and kept under short day conditions it will not flower.
Flowering will occur even when all the leaves are removed except one leaf. If a cocklebur plant is defoliated and kept under long day conditions, it will not flower. If one of its leaves is exposed to short day condition and rest are in long day condition, flowering will occur.
Question 10.
Give a brief account on Pr grammed Cell Death (PCD).
Answer:
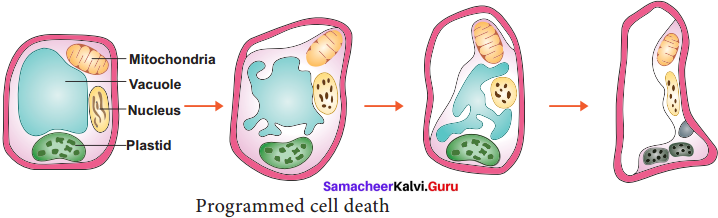
Senescence is controlled by plants own genetic program and death of the plant or plant part consequent to senescence is called Programmed Cell Death. In short senescence of an individual cell is called PCD. The proteolytic enzymes involving PCD in plants are phytases and in animals are caspases. The nutrients and other substrates from senescing cells and tissues are remobilized and reallocated to other parts of the plant that survives.
The protoplasts of developing xylem vessels and tracheids die and disappear at maturity to make them functionally efficient to conduct water for transport. In aquatic plants, aerenchyma is normally formed in different parts of the plant such as roots and stems which encloses large air spaces that are created through PCD. In the development of unisexual flowers, male and female flowers are present in earlier stages, but only one of these two completes its development while other aborts through PCD.
Samacheer Kalvi 11th Bio Botany Plant Growth and Development Additional Questions & Answers
I. Choose T he correct answer (1 Mark)
Question 1.
Open form of the growth occurs in:
(a) leaves and flowers
(b) stem and root
(c) leaves and stem
(d) stem and flowers
Answer:
(b) stem and root
Question 2.
Bamboo is classified under:
(a) monocarpi c annual plants
(b) polycarpic perennials
(c) monocarpic perennials
(d) polycarpic annual plants
Answer:
(c) monocarpic perennials
Question 3.
Primary growth of the plant is due to the activity of:
(a) phloem parenchyma
(b) phloem meristem
(c) vascular cambium
(d) apical meristem
Answer:
(d) apical meristem
Question 4.
One single maize root apical meristem can give rise to more than:
(a) 17,500 hew cells per hour
(b) 18,500 new cells per hour
(c) 19,000 new cells per hour
(d) 500 new cells per hour
Answer:
(a) 17,500 hew cells per hour
Question 5.
Thickening and differentiation of cells take place during:
(a) elongation phase
(b) formative phase
(c) maturation phase
(d) flowering phase
Answer:
(c) maturation phase
Question 6.
When die total growth of a plant is plotted against time, the shape of the curve obtained is:
(a) hyperbolic curve
(b) ‘S’ shaped sigmoid curve
(c) linear curve
(d) none of the above
Answer:
(b) ‘S’ shaped sigmoid curve
Question 7.
The total growth of the plant consists of four phases in the following order.
(a) Log phase, lag phase, decelerating phase and maturation phase
(b) Log phase, lag phase, maturation phase and decelerating phase
(c) Lag phase, log phase, maturation phase and decelerating phase
(d) Lag phase, log phase, decelerating phase and maturation phase
Answer:
(d) Lag phase, log phase, decelerating phase and maturation phase
Question 8.
Internal factors, that influences the growth of the plant is:
(a) nutrition
(b) light
(c) C / N ratio
(d) oxygen
Answer:
(c) C / N ratio
Question 9.
Absence of light may lead to yellowish in color in plants and this is called:
(a) venation
(b) etiolation
(c) estivation
(d) vernation
Answer:
(b) etiolation
Question 10.
Differentiated cells, after multiplication again lose the ability to divide and mature to perform specific functions. This is called:
(a) plasticity
(b) differentiation
(c) dedifferentiation
(d) redifferentiation
Answer:
(d) redifferentiation
Question 11.
Indicate a plant growth regulator from the following:
(a) cytocin
(b) cytokinins
(c) acetic acid
(d) methylene
Answer:
(b) cytokinins
Question 12.
Some of the polyamines are known to behave like:
(a) growth inhibitors
(b) plant hormones
(c) flowering inhibitors
(d) fruit ripening agent
Answer:
(b) plant hormones
Question 13.
The activity of synergistic effect involves the activity of:
(a) auxin and gibberellins
(b) auxin and ethylene
(c) ABA and gibberellins
(d) none of the above
Answer:
(a) auxin and gibberellins
Question 14.
Phytohormones are usually produced to in tips of:
(a) root alone
(b) stem alone
(c) leaves alone
(d) root, stem and leaves
Answer:
(d) root, stem and leaves
Question 15.
The term auxin was first coined by:
(a) Charles Darwin
(b) Kogl
(c) F.W. Went
(d) Smith
Answer:
(c) F.W. Went
Question 16.
Indole Acetic Acid (IAA) is a:
(a) growth inhibitor
(b) hetero auxin
(c) root inhibitor
(d) synthetic auxin
Answer:
(b) hetero auxin
Question 17.
Indicate a synthetic auxin.
(a) Indole Acetic Acid
(b) Phenyl Acetic Acid
(c) Indole Butyric Acid
(d) Napthalene Acetic Acid
Answer:
(d) Napthalene Acetic Acid
Question 18.
Auxin has a similar chemical structure of:
(a) Indole acetic acid
(b) Napthalene acetic acid
(c) Phenyl acetic acid
(d) 2,4 – Dichloro phenoxy
Answer:
(a) Indole acetic acid
Question 19.
Auxin stimulates:
(a) transpiration
(b) respiration
(c) flowering
(d) none of the above
Answer:
(b) respiration
Question 20.
The term gibberllin was named by:
(a) Brain
(b) Yabuta
(c) Sumiki
(d) kurosawa
Answer:
(b) Yabuta
Question 21.
Who established the structure of gibberellic acid?
(a) Brain etal
(b) Kurosawa
(c) Cross et al
(d) Yabuta and Sumiki
Answer:
(c) Cross et al
Question 22.
Formation of seedless fruits without fertilization is induced by:
(a) auxin
(b) cytokinin
(c) ethylene
(d) gibberellin
Answer:
(d) gibberellin
Question 23.
Cytokinins inducing cell division was first demonstrated by:
(a) Haberlandt
(b) Charles Darwin
(c) Clarke
(d) Hubert
Answer:
(a) Haberlandt
Question 24.
Zeatin is first isolated from unripe grains of:
(a) paddy
(b) wheat
(c) maize
(d) com
Answer:
(c) maize
Question 25.
Indicate correct statements.
(i) Genes are intracellular factors for growth.
(ii) Temperature has no role in the growth of plant.
(iii) Oxygen has a vital role in the growth of plants.
(iv) CIN ratio of soil does not affect the growth of plant.
(a) (i) and (iv)
(b) (ii) and (iv)
(c) (i) and (iii)
(d) (ii) and (iii)
Answer:
(c) (i) and (iii)
Question 26.
Aspartic acid is classified under:
(a) freeauxin
(b) precursor of auxin
(c) chemical structure of auxin
(d) bound auxin
Answer:
(d) bound auxin
Question 27.
The stress phytohormones (Abscisic acid) was first isolated by:
(a) Linn et al
(b) Addicott et al
(c) Edward et al
(d) Stone and Black
Answer:
(b) Addicott et al
Question 28.
The chemical structure of abscisic acid resembles the structure of:
(a) indole Acetic Acid
(b) malanic acid
(c) carotenoid
(d) xanthophyll
Answer:
(c) carotenoid
Question 29.
Pick out the correct statement from the following:
(i) Abscisic acid is found abundantly inside the chloroplast of green cells.
(ii) ABA is a powerful growth promotor.
(iii) ABA is formed from pentose phosphate pathway.
(iv) ABA has anti-auxih and anti-gibberellin property.
(a) (i) and (iv)
(b) (i) and (ii)
(c) (ii) and (iii)
(d) (ii) and (iv)
Answer:
(a) (i) and (iv)
Question 30.
Abscisic acid induces male flower formation on female plants of:
(a) potato
(b) Cannabis sativa
(c) Vinca rosea
(d) Delomix regia
Answer:
(b) Cannabis sativa
Question 31.
Pea and barley are classified under:
(a) short day plants
(b) short long day plants
(c) long day plants
(d) long short day plants
Answer:
(c) long day plants
Question 32.
The term ‘photoperiodism’ was coined by:
(a) Miller and Amald
(b) Gamer and Allard
(c) Michael and Edward
(d) Darwin and Lamark
Answer:
(b) Gamer and Allard
Question 33.
Usually Xanthiumpensylvanicum will flower under:
(a) long day condition
(b) short long day condition
(c) photoneutral condition
(d) short day condition
Answer:
(d) short day condition
Question 34.
Phytochrome is a:
(a) reddish xanthophyll pigment
(b) bluish biliprotein pigment
(c) rodopsin pigment
(d) none of the above
Answer:
(b) bluish biliprotein pigment
Question 35.
Who found out the phytochrome in plants?
(a) Butler et al
(b) Michell et al
(c) Boumick et al
(d) Gamers and Allard
Answer:
(a) Butler et al
Question 36.
The term “vernalization” was first used by:
(a) Gamer
(b) Michell
(c) Lysenko
(d) Kawasacki
Answer:
(c) Lysenko
Question 37.
Pick out the wrong statement from the following:
(a) Vernalization increases the cold resistance of plants
(b) It increase the resistance of plants to fungal disease
(c) Vemalizatiqn increase the vegetative period of the plant
(d) It accelerates the plant breeding
Answer:
(c) Vemalizatiqn increase the vegetative period of the plant
Question 38.
In Oxalis, the seed viability ranges from:
(a) 10 to 15 years
(b) a few days
(c) more than 100 years
(d) upto 100 years
Answer:
(b) a few days
Question 39.
In apple and plum, the method of breaking seed dormancy involves the process of:
(a) impaction
(b) Scarification
(c) exposing to red light
(d) Stratification
Answer:
(d) Stratification
Question 40.
The proteolytic enzymes involved in – programmed cell death in plants are:
(a) phytochrome
(b) caspases
(c) phytaspases
(d) protolysis
Answer:
(c) phytaspases
II. Answer the following (2 Marks)
Question 1.
Define closed form of growth in plants.
Answer:
Leaves, flowers and fruits are limited in growth or of determinate or closed form growth.
Question 2.
What is meant by grand period of growth in plants?
Answer:
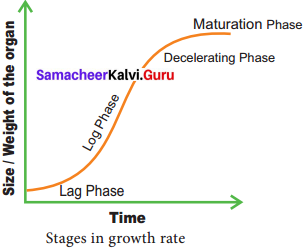
The total period from initial to the final stage of growth is called the grand period of growth. The total growth is plotted against time and ‘S’ shaped sigmoid curve (Grand period curve) is obtained.
Question 3.
Name the phases of growth in ‘S’ shaped growth curve.
Answer:
- Lag phase
- Log phase
- Decelerating phase
- Maturation phase
Question 4.
Define arithmetic growth rate in plant organ.
Answer:
If the length of a plant organ is plotted against time, it shows a linear curve and this growth is called arithmetic growth.
Question 5.
Distinguish between absolute growth rate and relative growth, rate.
Answer:
Absolute growth rate:
Increase in total growth of two organs measured and compared per unit time is called absolute growth rate.
Relative growth rate:
The growth of the given system per unit time expressed per unit initial parameter is called relative growth rate.
Question 6.
Define the term etiolation
Answer:
Light has its own contribution in the growth of the plant. Light is important for growth and photosynthesis. Light stimulates healthy growth. Absence of light may lead to yellowish in colour. This is called etiolation.
Question 7.
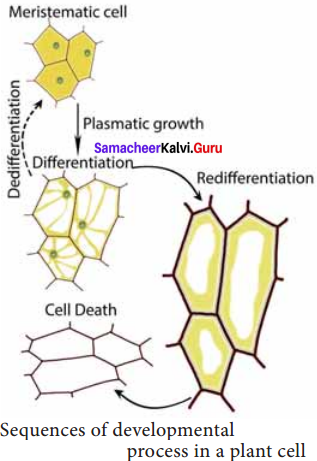
What is meant by redifferentiation of plant cells?
Answer:
Differentiated cells, after multiplication again lose the ability to divide and mature to perform specific functions. This is called redifferentiation, eg: Secondary xylem and Secondary phloem.
Question 9.
Mention any two synthetic auxins.
Answer:
- 2,4 – Dichloro Phenoxy Acetic Acid (2,4 – D)
- 2, 4, 5 – Trichloro Phenoxy Acetic Acid (2,4,5 – T)
Question 10.
Explain the synergistic effect of phytochromes.
Answer:
The effect of one or more substance in such a way that both promote each others activity, eg: Activity of auxin and gibberellins or cytokinins.
Question 11.
Name the natural auxins present in plants.
Answer:
- Indole Acetic Acid (IAA)
- Indole Propionic Acid (IPA)
- Indole Butyric Acid (IBA)
- Phenyl Acetic Acid (PAA)
Question 12.
Mention any two physiological effect of auxins in plant.
Answer:
- They promote cell elongation in stem and coleoptile.
- At higher concentrations auxins inhibit the elongation of roots but induce more lateral roots. Promotes growth of root only at extremely low concentrations.
Question 13.
Match the following.
| (i) Indole acetic acid | (a) bolting |
| (ii) Napthalene acetic acid | (b) anti-auxin |
| (iii) Gibberellins | (c) synthetic auxin |
| (iv) Abscisic acid | (d) Natural auxin |
Answer:
(i) – (d) Natural auxin
(ii) – (c) synthetic auxin
(iii) – (a) bolting
(iv) – (b) anti-auxin
Question 14.
Where do you find cytokinin hormone in plants?
Answer:
The distribution of cytokinin in plants is not as wide as those of auxin and gibberellins but found mostly in roots. Cytokinins appear to be translocated through xylem.
Question 15.
What is Richmond Lang effect?
Answer:
Application of cytokinin delays the process of aging by nutrient mobilization. It is known as Richmond Lang effect.
Question 16.
What is meant by non-climacteric fruits?
Answer:
All fruits cannot be ripened by exposure to ethylene. Such fruits are called nonclimacteric fruits and are insensitive to ethylene, eg: Grapes, Watermelon, Orange.
Question 17.
Why do you call Abscisic acid (ABA) as stress hormone?
Answer:
It inhibits the shoot growth and promotes growth of root system. This character protect the plants from water stress. Hence, ABA is called as stress hormone.
Question 18.
What is meant by short day plants?
Answer:
The plants that require a short critical day length for flowering are called short day plants or long night plants, eg: Tobacco, Cocklebur, Soybean, Rice and Chrysanthemum.
Question 19.
Write down the importance of photoperiodism in plants.
Answer:
- The knowledge of photoperiodism plays an important role in hybridisation experiments.
- Photoperiodism is an excellent example of physiological pre-conditioning that is using an external factor to induce physiological changes in the plant.
Question 20.
Define the term vernalization.
Answer:
Besides photoperiod certain plants require a low temperature exposure in their earlier stages for flowering. Many species of biennials and perennials are induced to flower by low temperature exposure (0°C to 5°C). This process is called Vernalization.
Question 21.
What is meant by Epigeal germination?
Answer:
During epigeal germination cotyledons are pushed out of the soil. This happens due to the elongation of the hypocotyl. eg: Castor and Bean.
Question 22.
Explain the term seed dormancy.
Answer:
The condition of a seed when it fails to germinate even in suitable environmental condition is called seed dormancy.
Question 23.
Define the term phytogerontology.
Answer:
The branch of botany which deals with ageing, abscission and senescence is called Phytogerontology.
Question 24.
Explain the term programmed cell death.
Answer:
Senescence is controlled by plants own genetic programme and death of the plant or plant part consequent to senescence is called Programmed Cell Death. In short senescence of an individual cell is called PCD.
Question 25.
Define the term “Abscission”.
Answer:
Abscission is a physiological process of shedding of organs like leaves, flowers, fruits and seeds from the parent plant body.
III. Answer the following (3 Marks)
Question 1.
Explain the different phases of growth in plants.
Answer:
There are three phases of growth.
1. Formative phase:
Growth in this phase occurs in. meristematic cells of shoot and root tips. These cells are small in size, have dense protoplasm, large nucleus and small vacuoles. Cells divide continuously by mitotic cell division. Some cells retain capability of cell division while other cells enter the next phase of growth.
2. Elongation Phase:
Newly formed daughter cells are pushed out of the meristematic zone and increases the volume. It requires auxin and food supply, deposition of new cell wall materials (intussusception), addition of protoplasm and development of central vacuole take place.
3. Maturation Phase:
During this stage cells attain mature form and size. Thickening and differentiation takes place. After differentiation, the cells do not grow further.
Question 2.
Draw the ‘S’ shaped growth curve and mark the different phases of growth
Answer:
Question 3.
Mention the internal factors, that affect the growth of plant.
Answer:
- Genes are intracellular factors for growth.
- Phytohormones are intracellular factors for growth, eg: auxin, gibberellin, cytokinin.
- C/N ratio.
Question 4.
What are the characteristic features of phytohormones?
Answer:
- Usually produced in tips of roots, stems and leaves.
- Transfer of hormones “from one place to another takes part through conductive systems.
- They are required in trace quantities.
- AH hormones are organic in nature.
- There are no specialized cells or organs for their secretion.
- They are capable of influencing physiological activities leading to promotion, inhibition and modification of growth.
Question 5.
List out the agricultural applications of auxins.
Answer:
- It is used to eradicate weeds, eg: 2,4 – D and 2,4,5 – T.
- Synthetic auxins are used in the formation of seedless fruits (Parthenocarpic fruit).
- It is used to break the dormancy in seeds.
- Induce flowering in Pineapple by NAA & 2,4 – D.
- Increase the number of female flowers and fruits in cucurbits.
Question 6.
Mention any three physiological effects of gibberellins.
Answer:
- It produces extraordinary elongation of stem caused by cell division and cell elongation.
- Rosette plants (genetic dwarfism) plants exhibit excessive inter modal growth when they are treated with gibberellins. This sudden elongation of stem followed by flowering is called bolting.
- Gibberellin breaks dormancy in potato tubers.
Question 7.
What are the uses of ethylene in agriculture?
Answer:
- Ethylene normally reduces flowering in plants except in Pine apple and Mango.
- It increases the number of female flowers and decreases the number of male flowers.
- Ethylene spray in cucumber crop produces female flowers and increases the yield.
Question 8.
What is meant by climacteric fruits?
Answer:
In most of the plants, there is sharp rise in respiration rate near the end of the development of fruit, called climacteric rise. Such fruits are called climacteric fruits. The ripening on demand can be induced in these fruits by exposing them to normal air containing about 1 ppm of ethylene. A liquid called ethephon is being used in fruit ripening as it continuously releases ethylene, eg: Tomato, Apples, Banana, Mango.
Question 9.
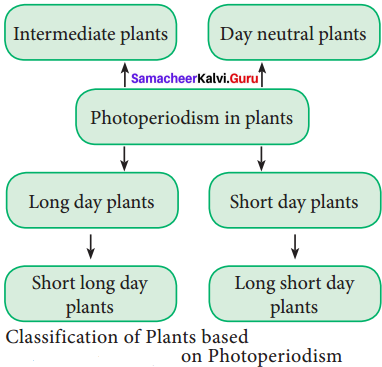
Give the classification of plants based on photoperiodism.
Answer:
Question 10.
Explain the term photoperiodic induction.
Answer:
An appropriate photoperiod in 24 hours cycle constitutes one inductive cycle. Plants , may require one or more inductive cycles for flowering. The phenomenon of conversion of leaf primordia into flower primordia under the influence of suitable inductive cycles is called photoperiodic induction.
eg: Xanthium (SDP) – 1 inductive cycle and Plantago (LDP) – 25 inductive cycles.
Question 11.
What are the practical applications of vernalization in plants?
Answer:
- Vernalization shortens’ the vegetative period and induces the plant to flower earlier.
- It increases the cold resistance of the plants.
- It increases the resistance of plants to fungal disease.
- Plant breeding can be accelerated.
Question 12.
Write down the internal factors, that affect seed germination.
Answer:
1. Maturity of embryo:
The seeds of some plants, when shed will contain immature embryo. Such seeds germinate only after maturation of embryo.
2. Viability:
Usually seeds remain viable or living only for a particular period. Viability of seeds range from a few days (eg: Oxalis) to more than hundred years. Maximum viability (1000 years) has been recorded in lotus seeds. Seeds germinate only within the period of viability.
3. Dormancy:
Seeds of many plants are dormant at the time of shedding.
Question 13.
Mention the factors causing dormancy of seeds.
Answer:
- Hard, tough seed coat causes barrier effect as impermeability of water, gas and restriction of the expansion of embryo prevents seed germination.
- Many species of seeds produce imperfectly developed embryos called rudimentary embryos which promotes dormancy.
- Lack of specific light requirement leads to seed dormancy.
- A range of temperatures either higher or lower cause dormancy.
- The presence of inhibitors like phenolic compounds which inhibits seed germination cause dormancy.
Question 14.
What are the significances of abscission?
Answer:
- Abscission separates dead parts of the plant, like old leaves and ripe fruits.
- It helps in dispersal of fruits and continuing the life cycle of the plant.
- Abscission of leaves in deciduous plants helps in water conservation during summer.
- In lower plants, shedding of vegetative parts like gemmae or plantlets help in vegetative reproduction.
IV. Answer the following (5 Marks)
Question 1.
Describe the geometric growth rate in plants with suitable diagram.
Answer:
Geometric growth rate:
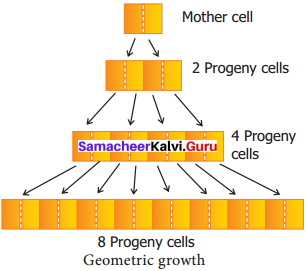
This growth occurs in many higher plants and plant organs and is measured in size or weight. In plant growth, geometric cell division results if all cells of an organism or tissue are active mitotically. eg: Round three in the given figure, produces 8 cells as 23 – 8 and after round 20 there are 220 = 1,048,576 cells. The large plant or animal parts are produced this way. In fact, it is common in animals but rare in plants except when they are young and small. Exponential growth curve can be expressed as,
W1 = W0ert
W1 = Final size (weight, height and number)
W0 = Initial size at the beginning of the period
r = Growth rate
t = Tittle of growth
e = Base of the natural logarithms
Here V is the relative growth rate and also a measure of the ability of the plant to produce new plant material, referred to as efficiency index. Hence, the final size of W1 depends on the initial size W0.
Question 2.
Describe the experiment to measure the increase in length of the stem tip using an arc auxanometer.
Answer:
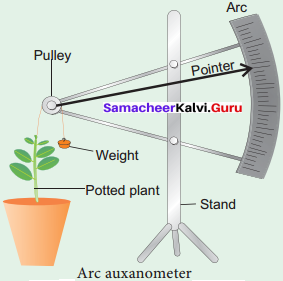
The increase in the length of the stem tip can easily be measured by an arc auxanometer which consists of a small pulley to the axis of which is attached a long pointer sliding over a graduated arc. A thread one end of which is tied to the stem tip and another end to a weight passes over the pulley tightly.
As soon as the stem tip increases in length, the pulley moves and the pointer slide over the graduated arc (Figure). The reading is taken. The actual increase in the length of the stem is then calculated by knowing the length of the pointer and the radius of the pulley. If the radius of the pulley is 4 inches and the length of pointer 20 inches the actual growth is measured as follows:

Question 3.
Write an essay on the phytochrome, Gibberelf ns in plants.
Answer:
1. Discovery:
The effect of gibberellins had been known in Japan since early 1800 where certain rice plants were found to suffer from ‘Bakanae’ or foolish seedling disease. This disease was found . by Kurosawa (1926) to be caused by a fungus Gibberella fujikuroi. The active substance was separated from fungus and named as gibberellin by Yabuta (1935). These are more than 100 gibberellins reported from both fungi and higher plants. They are noted as GA1, GA2, GA3 and so on. GA3 is the first discovered gibberellin. In 1938, Yabuta and Sumiki isolated gibberellin in crystalline form. In 1955, Brain et al., gave the name gibberellic acid. In 1961, Cross et al., established its structure.
2. Occurrence:
The major site of gibberellin production in plants is parts like embryo, roots and young leaves near the tip. Immature seeds are rich in gibberellins.
3. Precursors:
The gibberellins are chemically related to terpenoids (natural rubber, carotenoids and steroids) formed by 5 – C precursor, an Isoprenoid unit called Iso Pentenyl Pyrophosphate (IPP) through a number of intermediates. The primary precursor is acetate.
4. Chemical structure:
All gibberellins have gibbane ring structure.
5. Transport in plants:
The transport of gibberellins in plants is non-polar. Gibberellins are translocated through phloem and also occur in xylem due to lateral movement between vascular bundles.
6. Bioassay (Dwarf Pea. assay):
Seeds of dwarf pea are allowed to germinate till the formation of the coleoptile. GA solution is applied to some seedlings. Others are kept under control. Epicotyle length is measured and as such, GA stimulating epicotyle growth can be seen.
7. Physiological Effects:
It produces extraordinary elongation of stem caused by cell division and cell elongation.
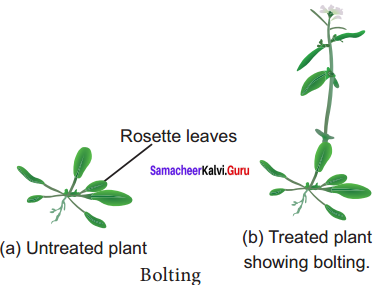
- Rosette plants (genetic dwarfism) plants exhibit excessive intermodal growth when they are treated with gibberellins. This sudden elongation of stem followed by flowering is called bolting.
- Gibberellin breaks dormancy in potato tubers.
- Many biennials usually flower during second year of their growth. For flowering to take place, these plants should be exposed to cold season. Such plants could be made to flower without exposure to cold season in the first year itself, when they are treated with gibberellins.
8. Agricultural role:
- Formation of seedless fruits without fertilization is induced by gibberellins eg: Seedless tomato, apple and cucumber.
- It promotes the formation of male flowers in cucurbitaceae. It helps in crop improvement.
Uniform bolting and increased uniform seed production. - Improves number and size of fruits in grapes. It increase yield.
- Promotes elongation of inter-node in sugarcane without decreasing sugar content.
- Promotion of flowering in long day plants even under short day conditions. .
- It stimulates the seed germination
Question 4.
What are their physiological effects of Abscisic acid in – plants and its role in agriculture?
Answer:
Physiological effects:
- It helps in reducing transpiration rate by closing stomata. It inhibits K+ uptake by guard cells and promotes the leakage of malic acid. It results in closure of stomata;
- It spoils chlorophylls, proteins and nucleic acids of leaves making them yellow.
- Inhibition of cell division and cell elongation.
- ABA is a powerful growth inhibitor. It causes 50% inhibition of growth in Oat coleoptile.
- It induces bud and seed dormancy.
- If promotes, the abscission of leaves, flowers and fruits by forming abscission layers.
- ABA plays an important role in plants dtiring water stress -and during drought conditions. It results in loss of turgor and closure of stomata,
- It has anti-auxin and anti-gibberellin property.
- Abscisic acid promotes senescence in leaves’ by causing loss of chlorophyll pigment decreasing the rate of photosynthesis and changing the rate of proteins and nucleic acid synthesis
Agricultural Role:
- In Cannabis sativa, induces male flower formation on female plants.
- Induction of flowers in short day plants.
- It promotes sprouting in storage organs like Potato.
- ABA plays an important role in plants during water stress drought conditions.
- It inhibits the shoot growth and promotes growth of root system. This character protect the plants from water stress. Hence, ABA is called as stress hormone.
Question 5.
Write an essay on the role of ethylene on plant physiology and agriculture.
Answer:
Almost all plant tissues produce ethylene gas in minute quantities.
1. Discovery:
In 1924, Denny found that ethylene stimulates the ripening of lemons. In 1934, R. Gane found that ripe bananas contain abundant ethylene. In 1935, Cocken et al., identified ethylene as a natural plant hormone.
2. Occurrence:
Maximum synthesis occurs during climacteric ripening of fruits and tissues undergoing senescence. It is formed in almost all plant parts like roots, leaves, flowers, fruits and seeds.
3. Transport in plants:
Ethylene can easily diffuse inside the plant through intercellular spaces.
4. Precursor:
It is a derivative of amino acid methionine, linolenic acid and fumaric acid.
5. Bioassay (Gas Chromatography):
Ethylene can be measured by gas chromatography. This technique helps in the detection of exact amount of ethylene from different plant tissues like lemon and orange.
6. Physiological Effects:
- Ethylene stimulates respiration and ripening in fruits.
- It stimulates radial growth in sterft and roof and inhibits linear growth.
- It breaks the dormancy of buds, seeds and storage organs.
- It stimulates formation of abscission zone in leaves, flowers and fruits. This makes the leaves to shed prematurely.
- Inhibition of stem elongation (shortening the intemode).
- In low concentration, ethylene helps in root initiation.
- Growth of lateral roots and root hairs. This increases the absorption surface of the plant roots.
- The growth of fruits is stimulated by ethylene in some plants. It is more marked in climacteric fruits.
- Ethylene causes epinasty.
7. Agricultural role:
- Ethylene normally reduces flowering in plants except in Pine apple and Mango.
- It increases the number of female flowers and decreases the number of male flowers.
- Ethylene spray in cucumber crop produces female flowers and increases the yield.
Question 6.
Explain the two hypothesis of explaining the mechanism of vernalization.
Answer:
Two main theories to explain the mechanism of vernalization are:
1. Hypothesis of phasic development:
According to Lysenko, development of an annual seed plant consists of two phases. First phase is thermostage, which is vegetative phase requiring low temperature and suitable moisture. Next phase is photo stage which requires high temperature for synthesis of florigen (flowering hormone).
2. Hypothesis of hormonal involvement:
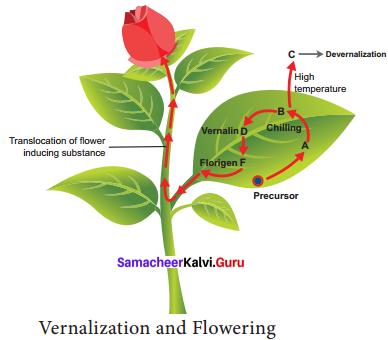
According to Purvis (1961), formation of a substance A from its precursor is converted into B after chilling. The substance B is unstable. At suitable temperature B is converted into stable compound D called Vemalin. Vernalin is converted to F (Florigen). Florigen induces flower formation. At high temperature B is converted to C and devemalization occurs.
Question 7.
Write an essay on the types of senescence, its physiology and the factors affecting senescence.
Answer:
1. Types of Senescence:
Leopold (1961) has recognised four types of senescence.
(a) Overall senescence:
This kind of senescence occurs in annual plants when entire plant gets affected and dies, eg: Wheat and Soybean. It also occurs in few perennials also, eg: Agave and Bamboo.
(b) Top senescence:
It occurs in aerial parts of plants. It is common in perennials, underground and root system remains viable, eg: Banana and Gladiolus.
(c) Deciduous senescence:
It is common in deciduous plants and occurs only in leaves of plants, bulk of the stem and root system remains alive, eg: Elm and Maple.
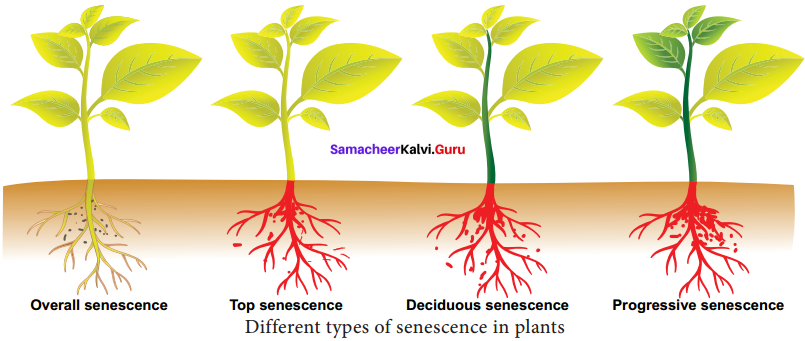
(d) Progressive senescence:
This kind of senescence is gradual. First it occurs in old leaves followed by new leaves f then stem and finally root system. It is common in annuals.
2. Physiology of Senescence:
- Cells undergo changes in structure.
- Vacuole of the cell acts as lysosome and secretes hydrolytic enzymes.
- The starch content is decreased in the cells.
- Photosynthesis is reduced due to loss of chlorophyll accompanied by synthesis and accumulation of anthocyanin pigments, therefore the leaf becomes red.
- There is a marked decrease in protein content in the senescing organ.
- RNA content of the leaf particularly rRNA level is decreased in the cells due to increased activity of the enzyme RNAase.
- DNA molecules in senescencing leaves degenerate by the increased activity of enzyme DNAase.
3. Factors affecting Senescence:
- ABA and ethylene accelerate senescence while auxin and cytokinin retard senescence.
- Nitrogen deficiency increases senescence whereas nitrogen supply retards, senescence. High temperature senescence but low retards senescence.
- Senescence is rapid in dark than in light.
- Water stress leads to accumulation of ABA leading to senescence.
Question 8.
Describe the methods of breaking dormancy of seeds in plants.
Answer:
The dormancy of seeds can be broken by different methods. These are:
1. Scarification:
Mechanical and chemical treatments like cutting or chipping of hard tough seed coat and use of organic solvents to remove waxy or fatty compounds are called as Scarification.
2. imp-action:
in some seeds water and oxygen are unable to penetrate micropyle due to blockage by cork cells. These seeds are shaken vigorously to remove the plug which is called Imp-action.
3. Stratification:
Seeds of rosaceous plants (Apple, Plum, Peach and Cherry) will not germinate until they have been exposed to well aerated, moist condition under low temperature (0°C to 10°C) for weeks to months. Such treatment is called Stratification.
4. Alternating temperatures: Germination of some seeds is strongly promoted by alternating daily temperatures. An alternation of low and high temperature improves the germination of seeds.
5. Light:
The dormancy of photoblastic seeds can be broken by exposing them to red light.
Solution To Activity
Textbook Page No : 164
Question 1.
Demonstration of phases of growth.
Answer:
To demonstrate and study the phases of growth, germinate a few seeds of bean on a circular filter paper soaked with water in a petridish. After two days of growth, select a few seedlings with straight radical of 2 to 3 cm length. Dry the surface of radical with a blotting paper and mark the radical from tip to base with at least 2 mm gap using water proof ink. Replace the seedlings in filter paper and observe further growth.
Observation:
The marked area in the radical will grow and increase in length and hence the marked area of 2mm is found to be grow beyond 2mm size due to the growth in the radical.
Textbook Page No: 169
Question 2.
Measurement of growth by direct method.
Answer:
Step 1: Take ordinary scale.
Step 2: Measure ground stem up to the growing point of the plant.
Step 3: Use Indian ink and mark at regular intervals to measure the length of root, stem, and girth of the trunk.
Observation:
At regular intervals measure the increase in length and girth of the trunk and it can be observed that the length of the root, stem and girth of the trunk increased with the increase in the period of growth.
Believing that the Samacheer Kalvi 11th Biology Book Solutions Questions and Answers learning resource will definitely guide you at the time of preparation. For more details about Tamilnadu State 11th Bio Botany Chapter 15 Plant Growth and Development textbook solutions, ask us in the below comments and we’ll revert back to you asap.








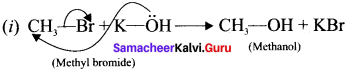
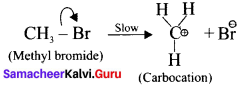
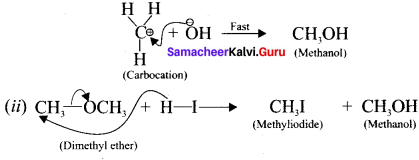
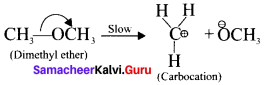
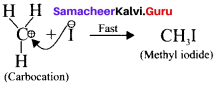
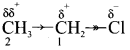
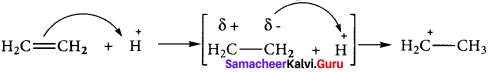
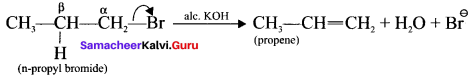



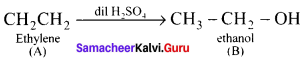
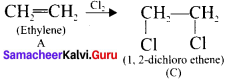
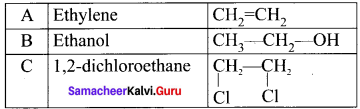
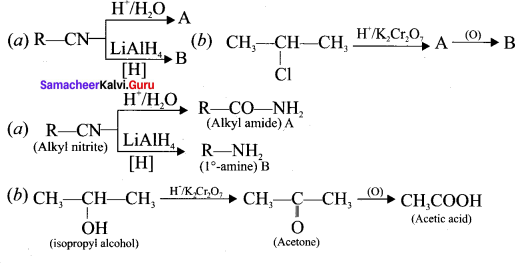
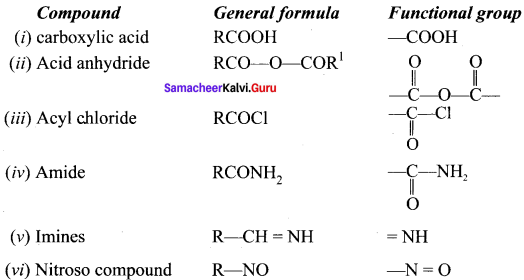
 is ……….
is ……….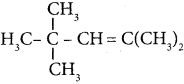 is ………
is ………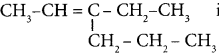 is ………
is ………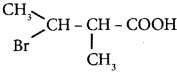 is ………
is ………
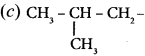
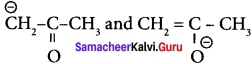 are ………
are ………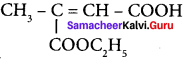 is 3-carbethoxy -2- butenoicacid.
is 3-carbethoxy -2- butenoicacid.
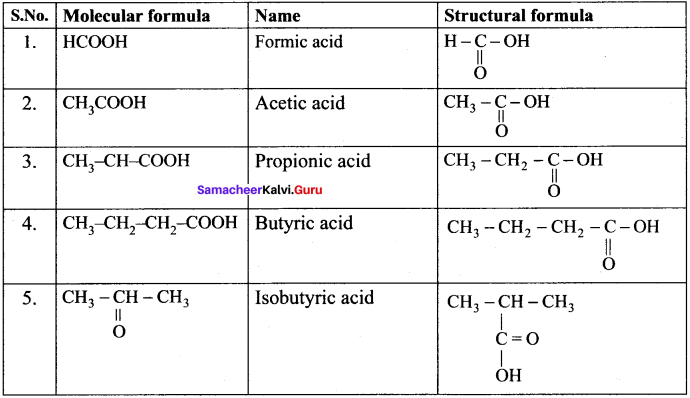
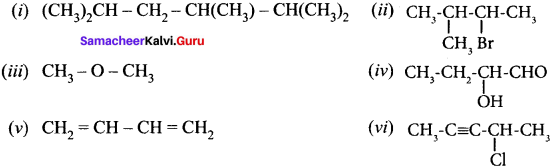
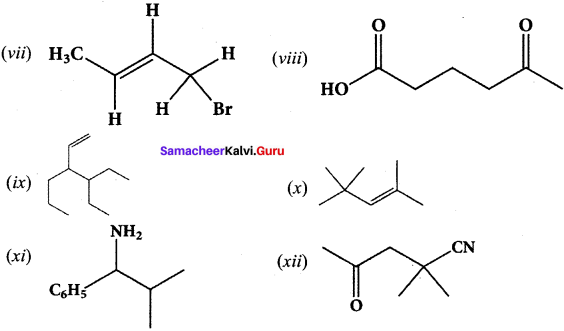
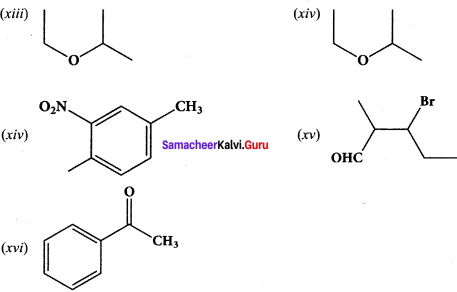







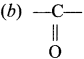

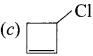
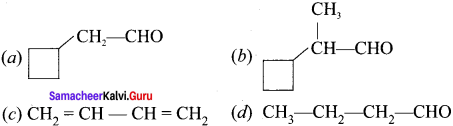
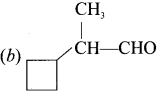

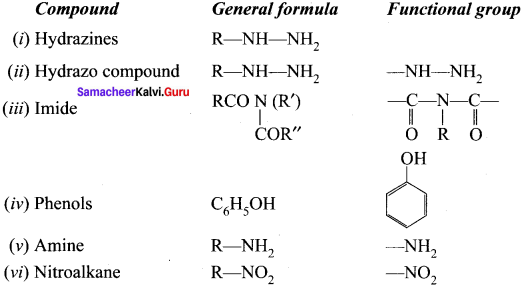
 ……..
……..
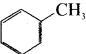 is an example of ………
is an example of ………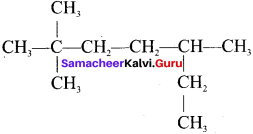 is …….
is …….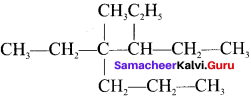 is ……..
is ……..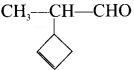
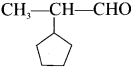 is …………
is ………… are called ……….
are called ……….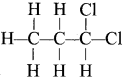
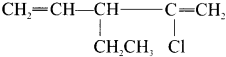 name of this compound is ………..
name of this compound is ………..
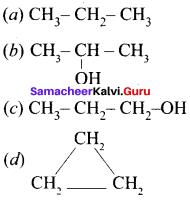

 is an aromatic benzenoid compound.
is an aromatic benzenoid compound.
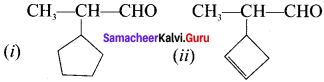

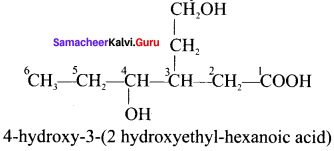
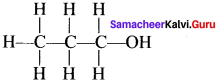
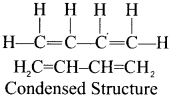
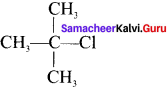

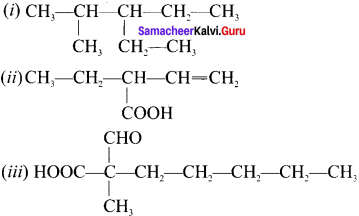
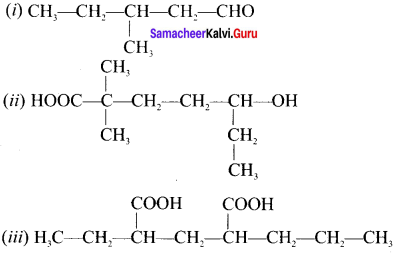
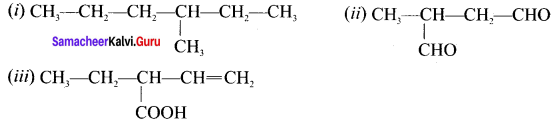
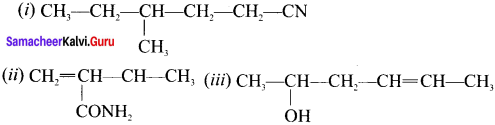
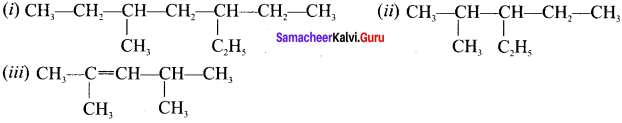
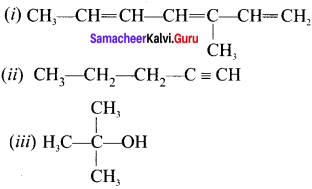
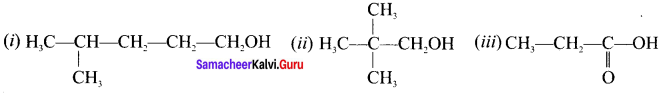

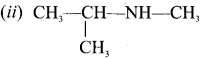

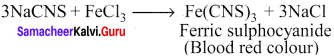





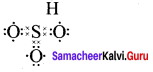
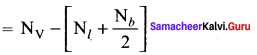
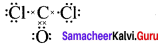
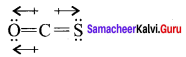


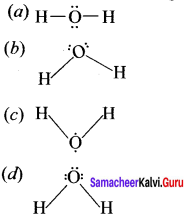
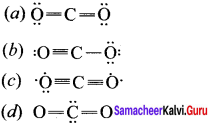
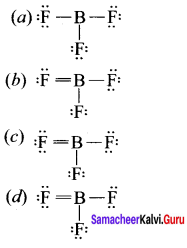
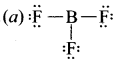
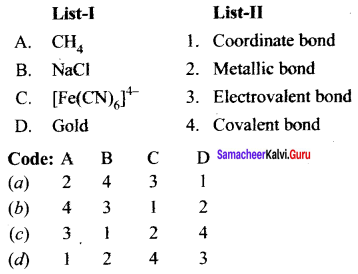
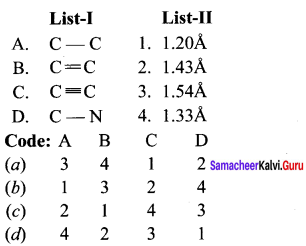
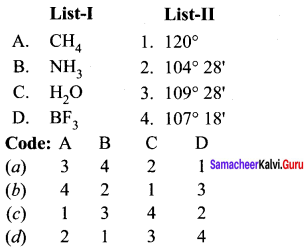
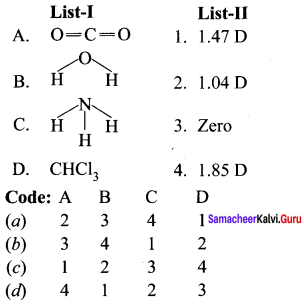


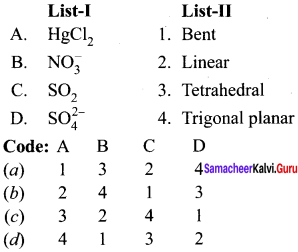
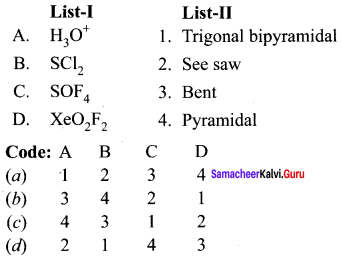
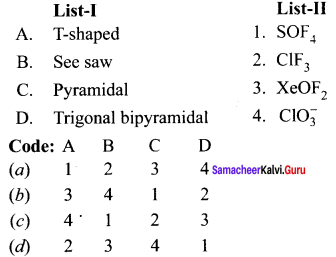
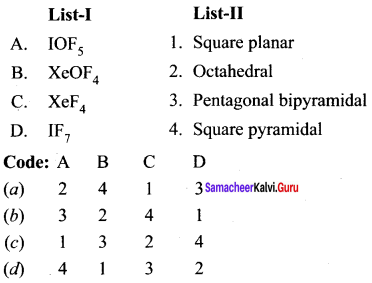
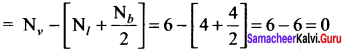
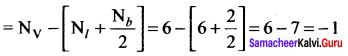
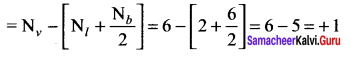
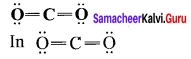
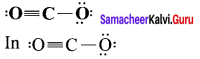
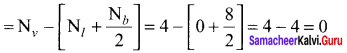
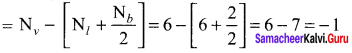
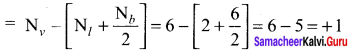
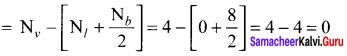
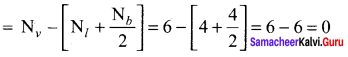
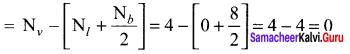
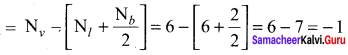
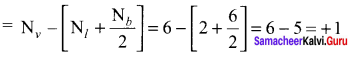
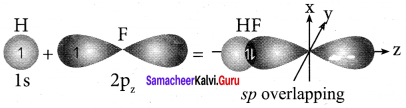




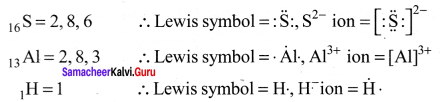
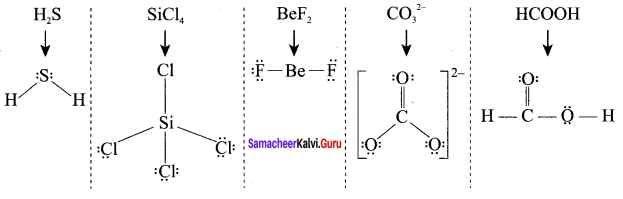
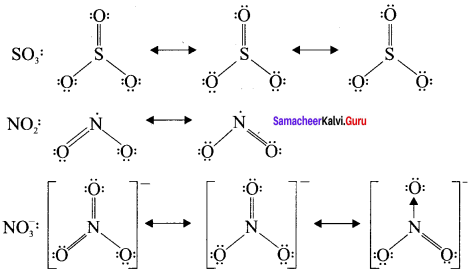
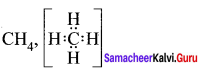
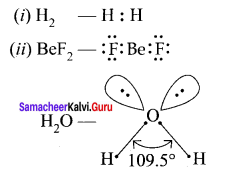
 there are two bond pairs and two lone pairs.
there are two bond pairs and two lone pairs.