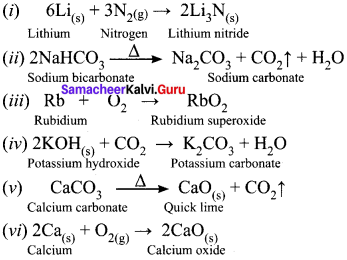
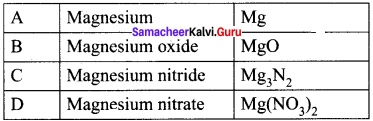
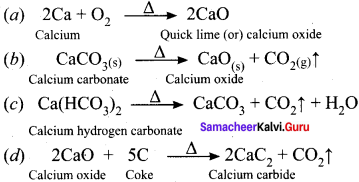
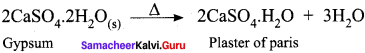
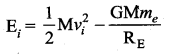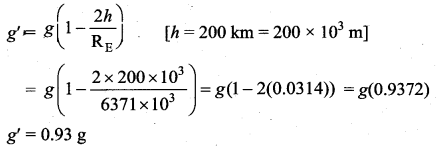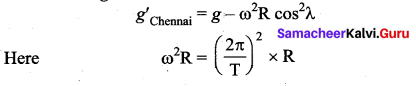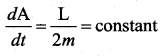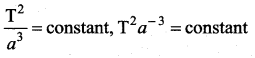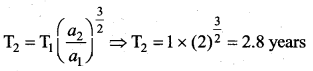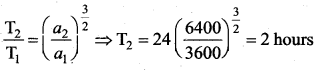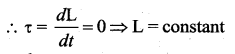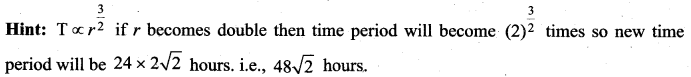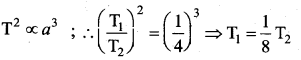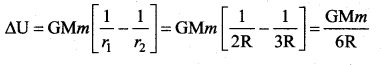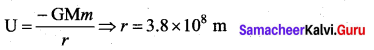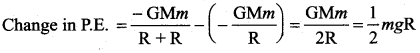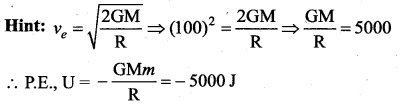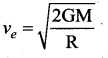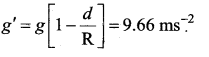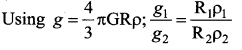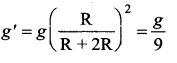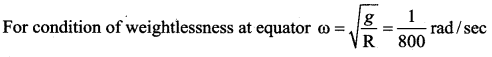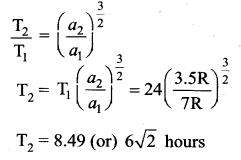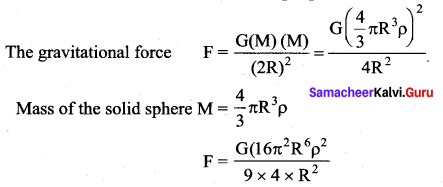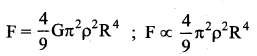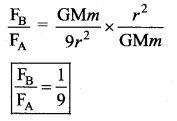Students who are interested in learning of 11th English Supplementary Chapter 5 The Singing Lesson Questions and Answers can use Tamilnadu State Board Solutions of 11th English Chapter Wise Pdf. First check in which chapter you are lagging and then Download Samacheer Kalvi 11th English Book Solutions Questions and Answers Summary, Activity, Notes Chapter Wise. Students can build self confidence by solving the solutions with the help of Tamilnadu State Board English Solutions. English is the scoring subject if you improve your grammar skills. Because most of the students will lose marks by writing grammar mistakes. So, we suggest you to Download Tamilnadu State Board 11th English Solutions according to the chapters.
Tamilnadu Samacheer Kalvi 11th English Solutions Supplementary Chapter 5 The Singing Lesson
Check out the topics covered in Supplementary Chapter 5 The Singing Lesson Questions and Answers before you start your preparation. Improve your grammar skills with the help of Samacheer Kalvi 11th English Book Solutions Questions and Answers pdf links. The solutions for Tamilnadu State Board 11th English Textbook are prepared by the English experts. So, if you follow Tamilnadu State Board Solutions 11th English Textbook Solutions you can cover all the topics in Supplementary Chapter 5 The Singing Lesson Questons and Answers. This helps to improve your communication skills.
Warm up
Question 1.
What are all the factors that influence our moods?
Human beings are bundles of emotions, A small angry look from a friend, a scolding from a teacher one admires, a taunt from mom could influence our moods the whole day.
Question 2.
How do you behave under the spells of different moods?
Answer:
When good things keep happening, we are happy. If we don’t get an easy question paper or the expected questions don’t appear we feel quite upset. If a close friend becomes angry, instead of analysing what caused it, we feel dejected. When centum is the goal, even 99% of marks disappoints us.
Question 3.
Do you think it is important not to be swayed by every passing mood?
Answer:
Yes, we should not be swayed by every passing emotions. But we are the sum total of our experiences. What ever bitter sweet experiences that occur do influence us. Latest researches . say that the food we eat, weather, clothes, the colour around us and punishing work schedules can adversely affect our moods. It would be ideal if we don’t allow external circumstances to influence our equanimity of mind and the ability to stay focused on our goals.
Question 4.
Suggest some ways by which we can maintain a calm temperament under all circumstances.
Answer:
Early morning walks, meditation and the practice of treating both success and failure, joy and sorrow with the same composure will naturally increase our life span on this planet. Listening to good music and reading good books, not only text books, can drastically reduce unpleasant stress. Thus we can maintain a calm temperament at all occasions.
Samacheer Kalvi 11th English The Singing Lesson Textual Questions
A. Based on your understanding of the story, answer the following questions in about 30 – 50 words each.
Question 1.
What was the knife that Miss Meadows carried with her?
Answer:
Miss. Meadows had received a letter from Mr. Basil calling off the marriage. She deemed it a kind of personal failure. Her anger and disappointed became despair. She carried cold despair buried deep in her heart like a wicked knife.
Question 2.
What kind of relationship existed between Miss Meadows and the Science Mistress?
Answer:
Both hated each other. Science Miss gave her a “Sugary smile” concealing her hostility. But Miss. Meadows enquiries responded to her deception always with a cold grimace.
Question 3.
Why was Miss Meadows upset and dejected?
Answer:
Mr. Basil had written a disquieting letter after his engagement with her. He had claimed that he was not a “Marrying man”. The thought of marriage gave him a feeling of disgust. He had struck out the word disgust and replaced it with ‘regret’ to lessen the hurt. So, Miss. Meadows was upset and dejected.
Question 4.
How would Miss Meadows usually treat Mary? How did her behaviour towards the girl change that day?
Answer:
Usually she would receive the flower from her favourite pupil Mary Beazley. She would tuck it in her belt with great tenderness and give a smile to her. The music class would start with a joyful note.
Question 5.
Why had Miss Meadows chosen ‘A Lament’ as the lesson that particular day?
Answer:
Miss. Meadows had chosen “A lament” as a lesson for that particular day. She was only in a mood to lament her broken engagement and shattered dreams. The choice of the music lesson reflected her real mood of dejection and despondency.
Question 6.
What brought agony to the girls during the music lessons?
Answer:
During the music lesson, Miss. Meadows did not show any warmth. She was icy cold and mechanical in her instructions. Children could easily realize that Miss. Meadow was in a wax. Miss. Meadow totally ignored the chrysanthemum from her favourite pupil. She also did not respond to her greeting. This sent tremors across the class. Young ones quickly understood the unstated message that music Miss was in one of her worst moods confirming their guess, she gave them “a lament” to practice.
The lesson was devoid of any warmth and joy. The lyrics of the song was so gloomy that children entered the world of unnatural agony and despair.
Question 7.
Bring out the substance of Basil’s letter to Miss Meadows.
Answer:
The content of Basil’s letter read, “I feel more and more strongly that our marriage would be a mistake. Not that I don’t love you. I love you as much as it is possible for me to love any woman but, truth to tell, I have come to the conclusion that I am not a marrying man and the idea of settling down fills me with nothing but – the word “disgust” was scratched out and “regret” written over the top.
Question 8.
Why did Miss. Wyatt summon Miss. Meadows to her room?
Answer:
A telegram addressed to Miss. Meadows was received at the school’s office. The Head mistress could not fathom the content of the Telegram. Believing that the telegram must be a harbinger of a tragedy, the Headniistress Miss. Wyatt summoned Miss. Meadows to her room.
Question 9.
How did Miss. Meadows express her joy, when she returned to the music class?
Answer:
Miss. Meadows changed the song for the children. She asked them to sing a joyful song beginning with flowers o’er laden. When she found that some children were still stuck up in the despondent mood, she reprimanded them. She told the girls, don’t look so doleful. It ought to sound warm, joyful and eager. And this time Miss. Meadow’s voice dominated the voices of all the little angels in her classroom. It was deep and glowing with a cheerful expression.
Question 10.
Briefly explain the cause of Miss Meadows’ joy at the end.
Answer:
Contrary to the expectation of Miss. Wyatt, the telegram was from Basil. It was an apology and reconciliatory in nature. In the telegram, her fiance had asked her to ignore the letter written when he must have been mad. A few hours before, she was the embodiment of disappointment and self-pity. The telegram had restored her joy. She could gather the pieces of her shattered dreams and hopes and built them anew. She was so happy that Miss. Wyatt’s warnings fell into deaf ears.
Vocabulary
A. Note the following words from the story. They all refer to different ways of walking. Find out their meanings and use each of them in meaningful sentences of your own. Refer a thesaurus and add a few more to the list.
(a) trod (b) fluttered (c) hurried (d) skipped (e) strode (f) sped
(a) trod – walked, stepped, strode, went
(b) fluttered – hovered, danced, flitted, flapped, oscillated, twitched, vibrated, flickered
(c) hurried – went fast, hastened, sped, charged, sprinted, chased, scampered, galloped, scrambled.
(d) skipped – capered, bobbed, bounded, jumped, leapt, gambolled,’frisked, romped
(e) strode – marched, trod, paced, stalked, dashed, ran, flaunted, joggled, tramped, rushed,zoomed
(f) sped – hurried, rushed, zipped, spurred, hurtled, sailed, hastened, quickened
B. Complete the mind map given below and write a brief summary of the story in your own words.
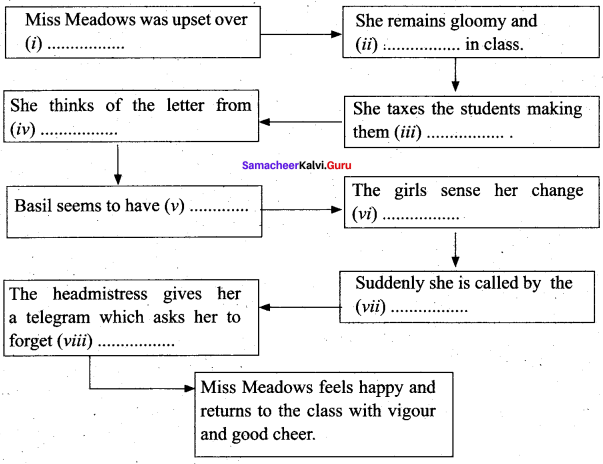
Answer:
(i) a letter from Basil breaking the engagement
(ii) irritable
(iii) sing a lament
(iv) Basil as a knife piercing her heart
(v) deserted her
(vi) of mpod
(vii) Head mistress Miss. Wyatt
(viii) the letter
A brief summary:
Miss Meadows was upset over a letter from Basil breaking the engagement. She remains gloomy and irritable in class. She taxes the students making them sing a lament. She thinks of the letter from Basil as a knife piercing her heart. Basil seems to have deserted her. Suddenly she is called by Head mistress Miss. Wyatt. The headmistress gives her a telegram which asks her to forget the letter. Miss Meadows feels happy and returns to the class with vigour and good cheer.
C. Answer the following questions in a paragraph of about 150 words each.
Question 1.
Describe Miss Meadows’ mood before and after receiving the telegram. How did it affect her class?
Answer:
Miss. Meadow was heart-broken. The letter written by Basil had pierced her heart and she was bleeding. Her hatred and anger became a knife and she carried it with her. Her icy cold response to science Miss demonstrates it. She is least bothered about the tender feelings of young children who look at her face all time for a friendly nod or smile of approval. Her favourite pupil Mary Beazley is baffled at her treatment of the chrysanthemum she had brought with so much love. The choice of the song “A lament” perfectly jells well with her worst mood. She is in fact in her heart lamenting over the loss of love, trust and future hopes. She is unnecessarily severe with young children forcing them to redo the singing which drives them to despair, pain and tears they manage to stifle.
After she receives the telegram from Basil apologizing for his insane letter, her mood changes to joy. She takes the chrysanthemum and keeps it close to her lips to conceal her blush. She goads the children to sing a song of joy congratulating some one for success. She persuades them to show warmth in their voices. Her warm and lively voice dominates the tremulous voices of the young ones. The young ones now realize that Miss Meadow who was in a wax earlier is now in her elements.
“My moods don’t just swing – they bounce, pivot, recoil, rebound, oscillate, fluctuate and occasionally PIROUETTE. ”
Question 2.
‘The only difference between a good day and a bad day is your attitude.’Relate this to a real life experience you have had. Share your thoughts in class.
Answer:
It is true that attitude makes or mars things. I was waiting for my Std X public exam resuls. I had put in 10 hours of work everyday. I was an average boy but I hoped to join the top five any time. The results were scheduled to be out the next morning. I have no internet connectivity at home. But one of my friends had it. His name is Murali. His father had come to my home. When I was out, he had told my parents I had failed in one subject. When I returned home, I found both my parents upset. I asked them what was wrong. They said it was a bad day. I asked them to tell me what was in their mind. They asked me to take things as they come by.
I didn’t understand. My dad told philosophically that failure is a stepping stone to success. Still they were not open with me. When I went out for tea, the local newspapers carried Std X public exam results. To my joy, I had passed with a first class. When I broke the news, my parents disclosed what had upset them. If they had just not reacted to the wrong information, their natural joy would not have been robbed. Now that they knew the information, they were over joyed. I just wondered at the human capacity to go down in misery and bounce back to extreme joy just by a turn of events. The best course of action to be happy all the time would be to. retain the key to our happiness and never give up on our joy to any external event.
“Don’t take it on yourself to repay a wrong. Trust the Lord and He will make it right.”
Question 3.
You are busy getting ready for school. You receive a Whats App message from your best friend, saying that he/she is very upset over the fight you had yesterday and doesn’t want to talk to you any more. This distresses you as she sounds very firm. However, today is a big day at school with two tests lined up. What will be your state of mind? How will you handle this situation?
Answer:
I always remember an anecdote. Kannadasan has recounted this anecdote. A temple elephant was proceeding to the temple. It’s mahout had washed him and applied sandal paste and holy ash on his forehead. Passerby greeted him like a God. As he was walking majestically, he was followed by a she elephant. A pig crossing the male elephant told its wife, “You see how the elephant was scared of me and gave way”. Overhearing the arrogant words of the pig, “The ‘she-elephant’ asked the ‘male elephant’ if it was true. The gentle animal smiled wisely and said, “I always focus on my goal.
We are on our way to a holy place. Even if I stamp on the pig by mistake, he would die. But I need to return to the tank for another wash.” “In life we need to avoid confrontation to ensure continuous progress in the chosen path. When I am a student, academics is quite important. Friendship is also important.
If a friend gets upset and if she really values my friendship, I can always say sorry and bring her around after the examination. If she is pig-headed and refuses to give up arrogance or anger, I will tell her I shall pray for her and move on. I will definitely find some one worthy of my true friendship. In reality, true friends, can’t be angry for long with each other. Realizing the value of true friends .
I’ll send a message wishing her the very best of luck for her exam and promise to sort out the issue in the evening. Nothing needs to be taken as a permanent failure in relationship or even in examination. I would like to remember the Chinese proverb “One can’t help birds of sorrow hover over one’s head. But one can prevent them from building nest in one’s head”.
“Never leave a true relationship for a few faults. Nobody is perfect; Nobody is correct. In the end… Affection is always greater than perfection. ”
Additional Question
Question 1.
Attempt a character sketch of Miss. Meadows.
Answer:
Miss. Meadows is a 30 year old lady. Mr. Basil a 25 year old young man gets engaged to her. When she is cherishing the dreams of a happy married life, a letter lands on her heart like a bombshell. It shatters her dreams. It pierces her heart. Being a sensitive lady, she feels her heart is bleeding. The contents of the letter keep haunting her memory. She is unable to focus in her music classes. The miraculous engagement was almost broken. The words, “our marriage could be a mistake” leaves her bleeding. She interpreted the scornful glance of science miss . as if she had known about the “break”. He had mentioned that he was not a marrying man”.
She wondered how she would react to the disclosure of the shattered engagement to the colleagues and the villagers. She even harboured the idea of leaving her job and go into hiding somewhere. As she is in a glooming mood, she doesn’t respond to the offer of chrysanthemum with a warm smile and thanks. She gives a ‘lament’ for practice. It is only when she receives a telegram of apology from her fiance her mood gets lifted. She flits on the wings of hope and sings a joyous song along with her students. She is a perfect example of ordinary mortals who are early hurt and quickly bounce back to hopeful life as well.
“Please don’t expect me to always be good, kind and loving. There are times when I will be cold, thoughtless and hard to understand.”
Additional Questions
I. Answer the following choosing from the options give below.
Question 1.
Miss. Meadows hugging the __________ stared in hatred at the science mistress.
(a) baton
(b) books
(c) bite
(d) knife
Answer:
(d) knife
Question 2.
Everything about Miss. Meadows was sweet, pale like __________
(a) money
(b) honey
(c) flower
(d) rose
Answer:
(b) honey
Question 3.
Science mistress was good at showing a __________ smile whenever she came across Miss. Meadows.
(a) honey
(b) sugary
(c) feigned
(d) deceptive
Answer:
(b) sugary
Question 4.
The story is set in __________ season.
(a) winter
(b) spring
(c) autumn
(d) rainy
Answer:
(c) autumn
Question 5.
One could witness an excitement in the school __________
(a) drowsy
(b) insipid
(c) gleeful
(d) sensational
Answer:
(c) gleeful
Question 6.
Everyday the presentation of a beautiful flower to Miss. Meadows by __________ her favourite has pupil become a ritual.
(a) Muriel
(b) Mr. Basil
(c) Rosy
(d) Mary Beazley
Answer:
(d) Mary Beazley
Question 7.
The little children in the music were thinking __________ is in a wax.
(a) Rowdy
(b) Meady
(c) Rosy
(d) Miss. Wyatt
Answer:
(b) Meady
Question 8.
The letter from Mr. Basil had __________ Miss. Meadows heart.
(a) gladdened
(b) soothed
(c) pierced
(d) embalmed
Answer:
(c) pierced
Question 9.
Mary’s __________ was totally ignored by Miss. Meadows.
(a) Rose
(b) Lilly
(c) Chrysanthemum
(d) apple
Answer:
(c) Chrysanthemum
Question 10.
The song chosen for practice in the music class was a __________
(a) love song
(b) lament
(c) joyous song
(d) hymn
Answer:
(b) lament
Question 11.
The rejection of the flower was a __________ moment in Mary Beazley’S school life.
(a) memorable
(b) unforgettable
(c) staggering
(d) astonishing
Answer:
(c) staggering
Question 12.
Nothing could be more __________ than the lament.
(a) important.
(b) beautiful
(c) tragic
(d) fabulous
Answer:
(c) tragic
Question 13.
The last time Mr. Basil had come to see Miss. Meadow, he had worn a __________ in his buttonhole.
(a) diamond
(b) pearl
(c) rose
(d) chrysanthemum
Answer:
(c) rose
Question 14.
Mr. Basil could not refuse the headmaster’s wife’s invitation for a __________ because he couldn’t afford to be unpopular.
(a) party
(b) lecture
(c) card game
(d) dinner
Answer:
(d) dinner
Question 15.
Basil had written to Miss. Meadows that their marriage would be a __________
(a) boon
(b) mistake
(c) marvel
(d) wonder
Answer:
(b) mistake
Question 16.
Mr. Basil’s previous letter was about a __________ book-case.
(a) sandal
(b) teak
(c) neem
(d) fumed-oak
Answer:
(d) fumed-oak
Question 17.
The tiny one who clung to the lament wriggled like __________ caught on a line.
(a) dogs
(b) cats
(c) elephant
(d) fishes
Answer:
(d) fishes
Question 18.
Miss. Meadows compliment with a strange, stony tone positively __________ the younger girls.
(a) encouraged
(b) boosted
(c) frightened
(d) pleased
Answer:
Question 19.
Miss. Meadows went on recalling the struck out word __________ in his letter.
(a) ‘pale’
(b) ‘white’
(c) ‘pleasing’
(d) ‘disgust’
Answer:
(d) ‘disgust’
Question 20.
Miss __________ was the school headmistress.
(a) Glory
(b) Mary
(c) Victoria
(d) Wyatt
Answer:
(d) Wyatt
Question 21.
Mrs. Wyatt really hoped for a news about a __________ through the telegram.
(a) marriage
(b) tragedy
(c) comedy
(d) practical joke
Answer:
(b) tragedy
Question 22.
Miss. Wyatt had sent for Miss. Meadow because __________ a had been received at the school office.
(a) letter
(b) money order
(c) book parcel
(d) telegram
Answer:
(d) telegram
Question 23.
On hearing the news of a telegram Miss. Meadows thought that __________ must have I committed suicide.
(a) Ryan
(b) Mary
(c) Mr. Basil
(d) Osborne
Answer:
(c) Mr. Basil
Question 24.
The telegram received the stress of Miss. Meadows but added to that of Miss __________
(a) Rose
(b) Beadle
(c) Mary
(d) Wyatt
Answer:
(d) Wyatt
Question 25.
Miss. Meadows had to struggle to lift the little girl from __________ spirit to a cheerful mood.
(a) doleful
(b) hateful
(c) disdainful
(d) cheerful
Answer:
(a) doleful
Question 26.
In order to indicate that she was her normal self again she took the __________ and held it to her lips.
(a) baton
(b) knife
(e) book
(d) chrysanthemum
Answer:
(d) chrysanthemum
Question 27.
The song after receiving the telegram sounded __________ , joyful and eager.
(a) cold
(b) distasteful
(c) mournful
(d) warm
Answer:
(d) warm
Question 28.
The fateful letter made Miss. Meadows even think of __________ her job and go into hiding.
(a) taking
(b) building
(c) resigning
(d) up scaling
Answer:
(c) resigning
Question 29.
After her brief visit to Miss. Wyatt’s room, dominated the students and it was glowing with __________
(a) pain
(b) bliss
(c) expression
(d) depression
Answer:
(c) expression
Question 30.
Miss Wyatt learnt that the telegram was Miss. Meadows’ __________
(a) dad
(b) fiance
(c) brother
(d) correspondent
Answer:
(b) fiance
II. Rearrange the sentences
Question 1.
(a) She did not say thanks with warmth to her favourite pupil for the flower.
(b) Most of the children realized with alarm that Miss. Meady was in a wax.
(c) Miss. Meadows looked dejected.
(d) Meadows, responding with a grimace went away and started her music.
(e) Science mistress greeted her with a sugary smile.
Answers:
(c) Miss. Meadows looked dejected.
(e) Science mistress greeted her with a sugary smile.
(d) Meadows, responding with a grimace went away and started her music.
(a) She did not say thanks with warmth to her favourite pupil for the flower
(b) Most of the children realized with alarm that Miss. Meady was in a wax.
Question 2.
(a) She was called to Miss. Wyatt’s room and given a telegram.
(b) Miss. Meadows, reflecting her despair, asked the children to sing “a lament”
(c) Miss. Meadows was upset over her fiance’s letter calling off the marriage
(d) After seeing the contents of the telegram, Miss. Meadows almost flew back to her class on the wings of hope and gave the class a joyful song to sing.
(e) Basil seemed to have deserted her.
Answers:
(c) Miss. Meadows was upset over her fiance’s letter calling off the marriage
(e) Basil seemed to have deserted her.
(b) Miss. Meadows, reflecting her despair, asked the children to sing “a lament”
(a) She was called to Miss. Wyatt’s room and given a telegram.
(d) After seeing the contents of the telegram, Miss. Meadows almost flew back to her class on the wings of hope and gave the class a joyful song to sing.
III. Identify the speaker
- “Isn’t it cold? It might be winter” – science mistress to Miss. Meadows
- “It is rather sharp – Miss Meadows to science mistress
- You look frozen – Science mistress to Miss. Meadows
- Oh! not quite as bad as that – Miss. Meadows to science mistress
- ‘sh – sh! gives’ – Mary Beazley to fellow students
- Silence, please! Immediately” – Miss Meadows to her student
- “Good morning Miss. Meadows” – Mary Beasley to music miss
- “Thank you Mary, How very nice! Turn to page 32” – Miss Meadows to Mary Beasley
- Page fourteen, please mark the accent – Miss. Meadows to the music class children
- What could have possessed him to write such a letter? – Monologue from Miss. Meadows
- The third line should be one crescendo – Miss. Meadows to her class
- “Away’ you must begin to die – to fade – until the listening ear” is nothing more than a whisper – Miss Meadows, to her music class students
- Well, Monica, what is it? – Miss Meadows to Monica
- Miss. Wyatt wants to see you in the mistress’s room. – Miss Monica to Miss. Meadows
- “I shall met you in honour to talk quietly while I am away,” – Miss to her students in music class
- “I sent for you just now because this telegram has come for you” – Miss Wyatt to Miss Meadows
- “A telegram for me, Miss. Wyatt” – Miss Meadows to Miss Wyatt
- “I hope it’s not bad news” – Miss Wyatt to Miss. Meadows
- “I do hope, it’s nothing serious” – Miss Meadows to Miss Wyatt
- Oh, no, thank you, Miss. Wyatt – Miss. Meadows to Miss Wyatt
- You’ve fifteen minutes more of your class … Miss Meadows, haven’t you? – Miss Wyatt to Miss Meadows.
- ”It’s from my fiance saying that… “ – Miss Meadows to Miss. Wyatt.
IV. Reading comprehension.
1. With despair – cold, sharp despair – buried deep in her heart like a wicked knife, Miss Meadows, in cap and gown and carrying a little baton, trod the cold corridors that led to the music hall. Girls of all ages, rosy from the air, and bubbling over with that gleeful excitement that comes from running to school on a fine autumn morning, hurried, skipped, fluttered by; from the hollow classrooms came a quick drumming of voices; a bell rang; a voice like a bird cried, “Muriel.”
And then there came from the staircase a tremendous knock-knock-knocking. Someone had dropped her dumb bells. The Science Mistress stopped Miss Meadows. “Good morning,” she cried, in her sweet, affected drawl. “Isn’t it cold? It might be winter.” Miss Meadows, hugging the knife, stared in hatred at the Science Mistress. Everything about her was sweet, pale, like honey. You would not have been surprised to see a bee caught in the tangles of that yellow hair. “It is rather sharp,” said Miss Meadows, grimly. The other smiled her sugary smile.
Question (a)
What is a baton used for?
Answer:
A baton is a long stick used for conducting a music orchestra.
Question (b)
What was the wicked knife?
Answer:
Sharp despair was the wicked knife.
Question (c)
Why was Miss. Meadows in a state of despair?
Answer:
Mr. Basic had written a letter calling of their engagement. So, Miss. Meadows was in a state of despair.
Question (d)
Why was the greeting of science mistress affected?
Science mistress did not have real feelings for Miss. Meadows. It was out of courtesy that she offered a sugary smile to Miss. Meadows and asked after the weather.
2. Forms Four, Five, and Six were assembled in the music hall. The noise was deafening. On the platform, by the piano, stood Mary Beazley, Miss Meadows’ favourite, who played accompaniments. She was turning the music stool. When she saw Miss Meadows, she gave a loud, warning “Sh-sh! Girls!” and Miss Meadows, her hands thrust in her sleeves, the baton under her arm, strode down the centre aisle, mounted the steps, turned sharply, seized the brass music stand, planted it in front of her, and gave two sharp taps with her baton for silence.
“Silence, please! Immediately!” and, looking at nobody, her glance swept over that sea of coloured flannel blouses, with bobbing pink faces and hands, quivering butterfly hair-bows, and music-books outspread. She knew perfectly well what they were thinking. “Meady is in a wax.” Well, let them think it! Her eyelids quivered; she tossed her head, defying them. What could the thoughts of those creatures matter to someone who stood there bleeding to death, pierced to the heart, by such a letter
Question (a)
Who was Miss. Meadows favourite pupil?
Answer:
Mary Beazley was Miss. Meadows’favourite pupil.
Question (b)
How did Mary alert the fellow students?
Answer:
On seeing Miss. Meadows, Mary alerted the fellow students saying,” sh – sh! girls”.
Question (c)
How did Miss. Meadows silence the music class?
Answer:
There was a brass music stand. Miss. Meadows gave two sharp taps with her baton to silence the music class.
Question (d)
According to Miss. Meadows, what were the children thinking about her on that bad day?
Answer:
Children were thinking that Miss. Meady is in a wax”.
Question (e)
What did the thoughts of children not affect Miss. Meadows?
Answer:
The letter from Mr. Basil had pierced her heart. She was bleeding to death. In such a state, Miss. Meadows could not possibly think about what the children were thinking about her.
The Singing Lesson About the Author

Kathleen Mansfield Murry (1888 – 1923) was a New Zealand short story writer who wrote under the pen-name Katherine Mansfield. She left New Zealand at the age of 19 and settled in the United Kingdom where she gained the friendship of great writers such as 0.11. Lawrence and Virginia Woolf. Bliss and The Garden Party were collections of short stories written by her. She wrote many poems and her collected letters were a great success.
The Singing Lesson Summary
This story depicts the fact that human moods are often influenced by experiences good or bad. The story revolves around the swings of mood experienced by Miss. Meadows and how it affected her work directly. Miss. Meadows, a music teacher aged 30, is engaged to 25 years old Basil. It was a huge surprise to everyone including the science teacher whom Miss. Meadows hates with all her heart. Suddenly she receives a disheartening letter from Basil that he is not a ‘marrying type of man’. The very idea of marriage gives him a feeling of “disgust”. But out of courtesy he had struck down the word disgust and written “regret”. After reading the letter Miss. Meady became gloomy. She had a feeling that her engagement was broken and it would soon come to the knowledge of everyone.
She would be a laughing stock. She will have to resign her job and go into hiding somewhere. This feeling of despair and disappointment had hurt her so much that she did not even accept the chrysanthemum from her favourite student Mary Beazley. A Chinese proverb says, “One cannot prevent. Under normal circumstances, she would have tucked the flower in her belt and returned a beaming smile to her favourite pupil. Miss. Meady made the children sing a lament creating an atmosphere of icy gloom befitting an occasion of mourning. She was very severe with young ones who didn’t evidence considerable pain and expression in her voice. A relief came to the drudgery of lament in the form of a telegram. Monica informed Miss. Meadows to meet the Headmistress. Miss. Meadows wondered if Mr. Basil had committed suicide. Her hands flew out in anxiety to collect the telegram from Miss. Wyatt.
Miss. Wyatt hoped it was bad news but out of politeness said, “I hope it is not bad news.” Miss. Meadows tore open the telegram. To her great relief it read, “Pay no attention to letter. Must have been mad, bought hat-stand today-Basil”. To answer the anxious query of Miss. Wyatt, Miss. Meadows blushed and said it was from her fiance. This happy turn of events upset Miss. Wyatt who disallowed telegrams on happy occasions. She reminded her of the 15 minutes left of her music class. Miss. Meadows ran all the way back to the music class. It appeared that she was flying on the wings of hope, love and joy. She picked up the deserted yellow chrysanthemum and held it to her lips to hide her smile.

Her mood suddenly switched over boundless joy. It reflected in the music lesson. She asked her pupils to turn to page 32 and sing the most joyous song the children had ever practised. Those who were blowing their nose from feigned sorrow and rigidity of the music, couldn’t suddenly move on to a joyful note. Miss. Meadow chided them for lack of feeling and expression.
Conclusion: The telegram restores her hopes, joy and faith in future, Many of us become prisoners of circumstances and over react to pinpricks in life. One should learn to be composed and take all kinds of information with a pinch of salt. Birds of sorrow hovering over one’s head. But one can prevent them from building nests in one’s head.
Textual:
accompaniments – music played to support an instrument, voice or group
aisle – a passage between rows of seats
drawl – slow, lazy way of talking
fiance – a man to whom one is engaged to be married
forte – a musical tone played loudly
grimace – expression of disgust on a person’s face of a music orchestra
tangles – a contused mass, twisted
Additional:
chided -rebuked
digust – revulsion
disallow – refuse
gloom – dejection
joyful – cheerful
joyous – happy
lament – expression of grief
regret – feel sad
relief – feeling of relaxation from tension
suicide – killing oneself
upset – pained
The main aim is to share the knowledge and help the students of 11th English to secure the best score in their final exams. Use the concepts of Samacheer Kalvi 11th English Book Solutions Supplementary Chapter 5 The Singing Lesson Questions and Answers in Real time to enhance your skills. If you have any doubts you can post your comments in the comment section, We will clarify your doubts as soon as possible without any delay.

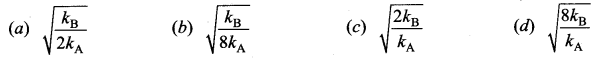
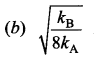
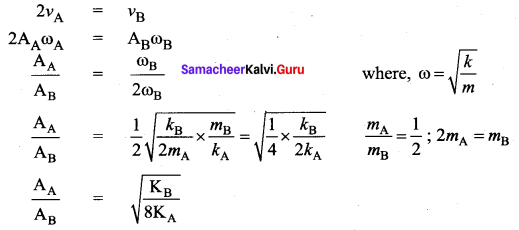

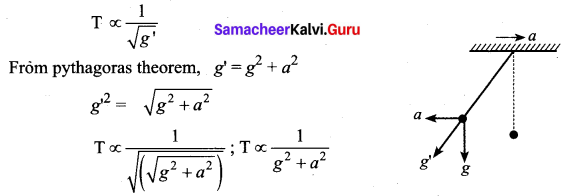

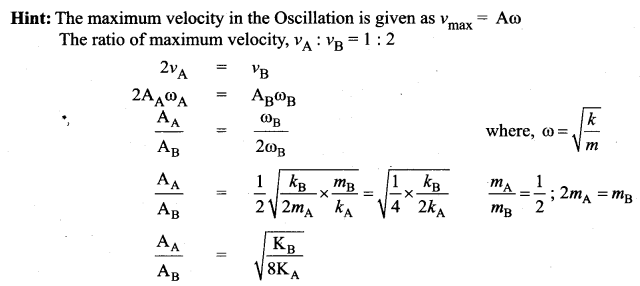

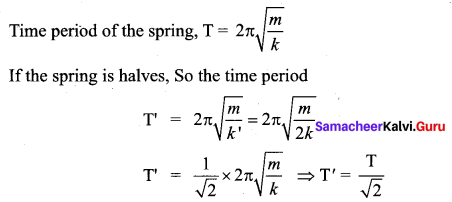
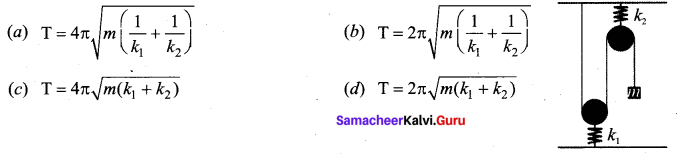
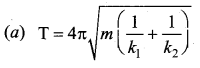
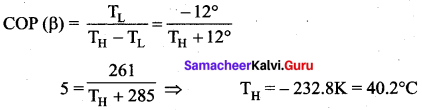
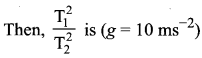 [IIT 2005]
[IIT 2005]
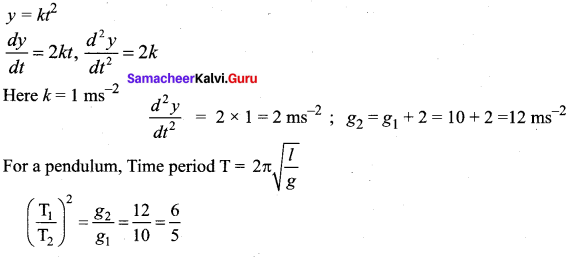

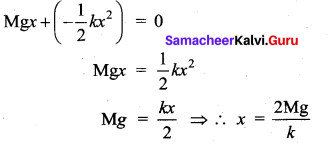
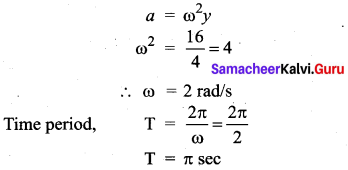
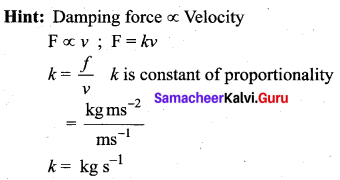

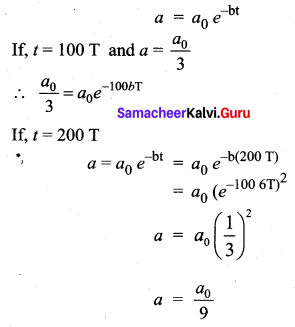

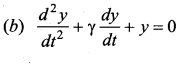
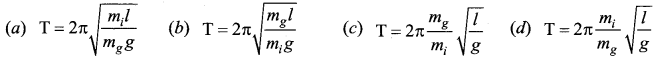
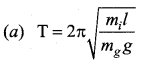
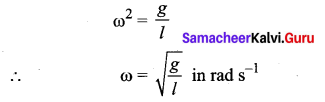
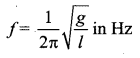
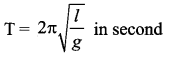
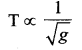




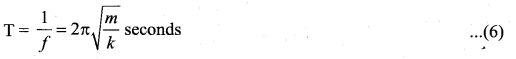
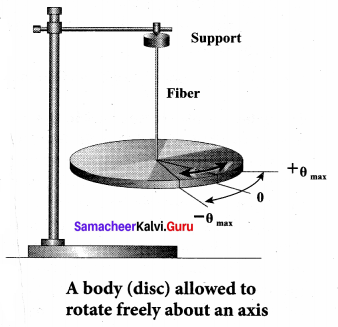

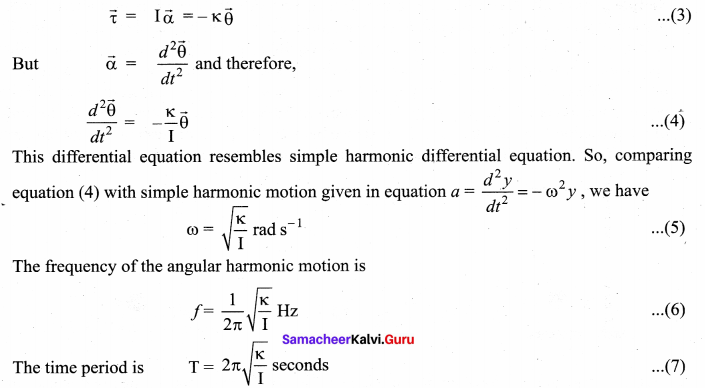
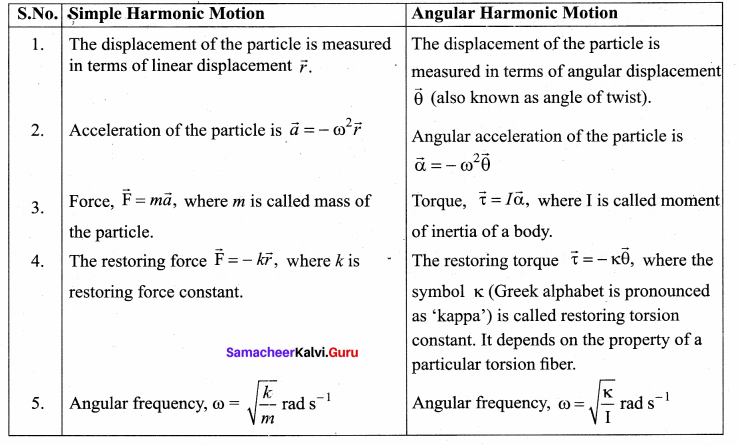




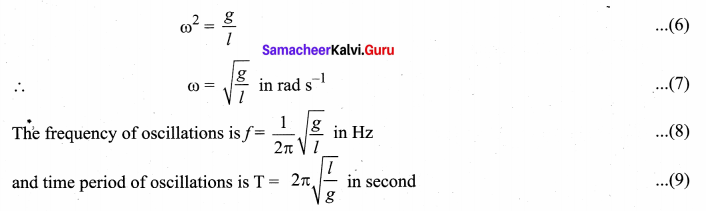
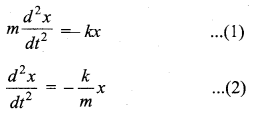
 >
>

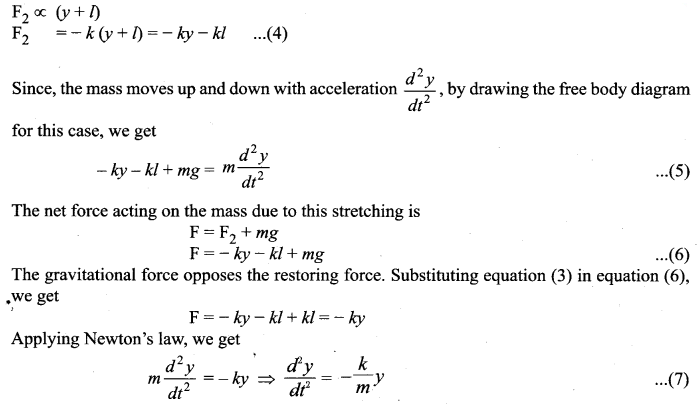

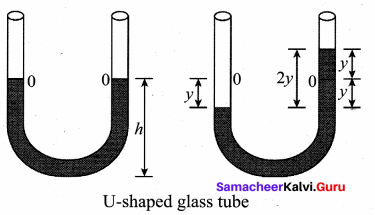
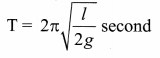
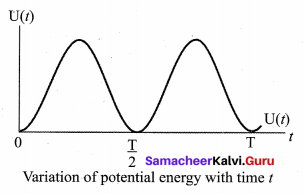
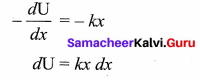
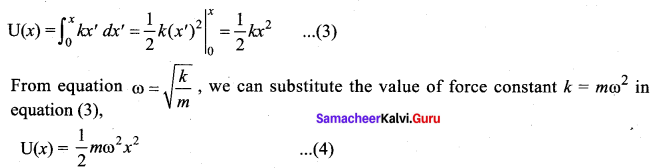
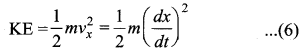
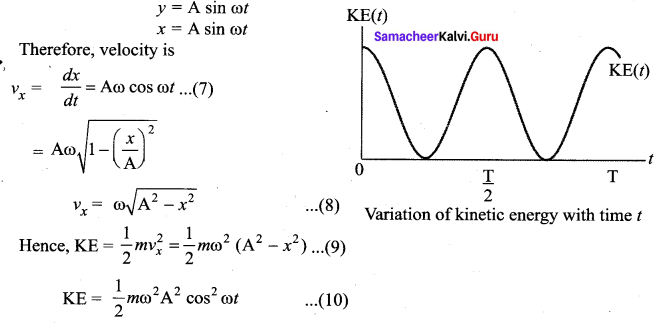
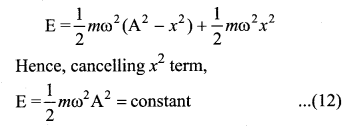
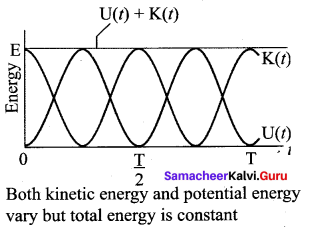
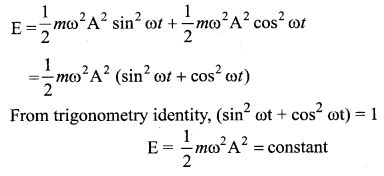

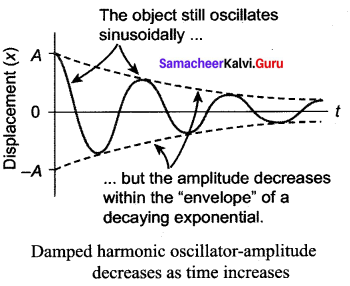
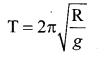
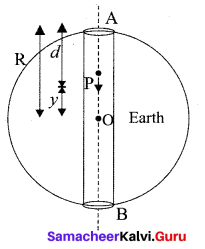
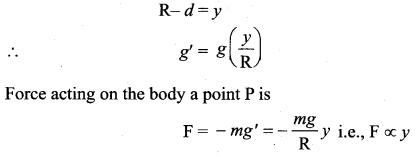

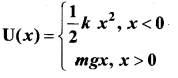
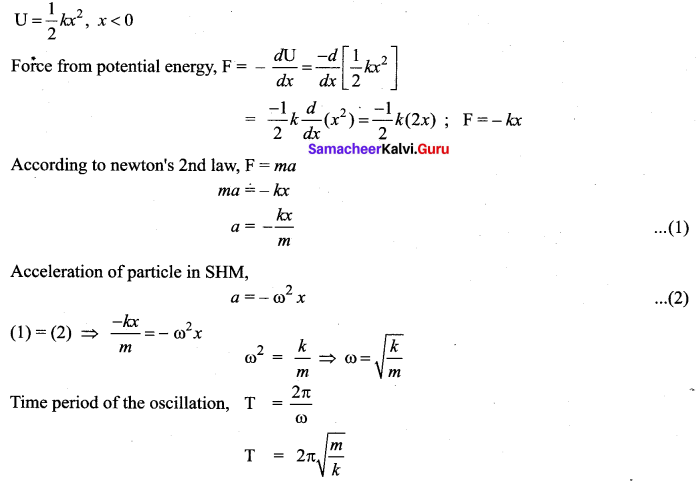
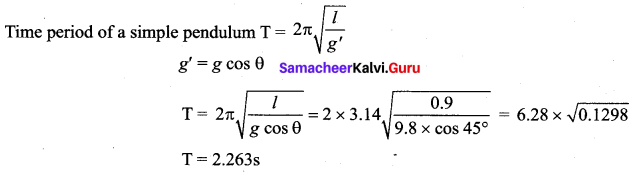
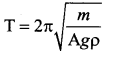
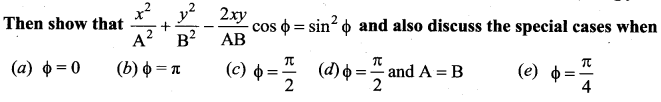
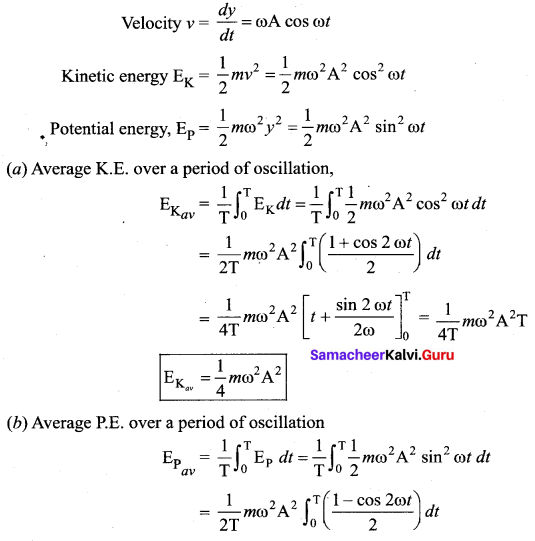
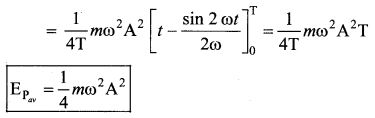
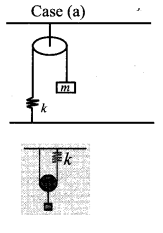
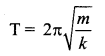


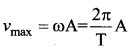
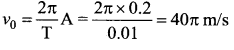
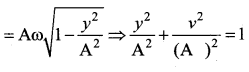
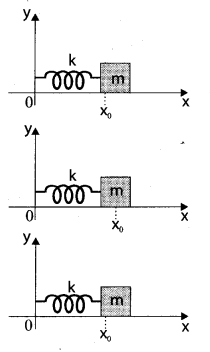
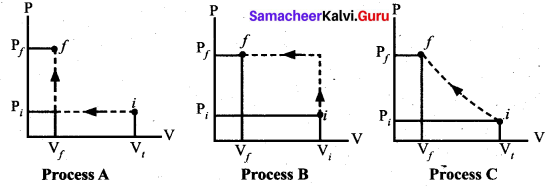
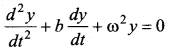 represents the equation of motion for a ……. vibration
represents the equation of motion for a ……. vibration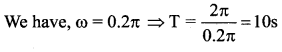

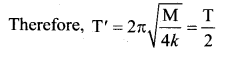

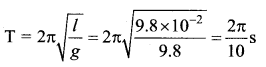
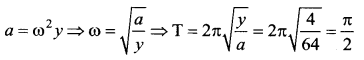

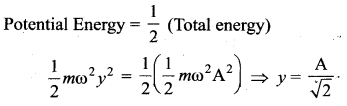

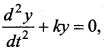 > where k is a positive constant. The time period of motion is given by …….
> where k is a positive constant. The time period of motion is given by …….
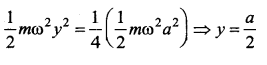
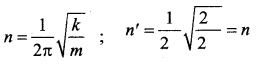
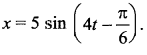 The velocity of the particle when its displacement is 3 units is …….
The velocity of the particle when its displacement is 3 units is …….
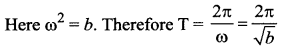

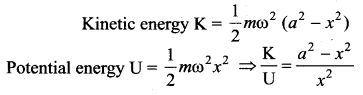
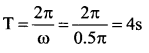
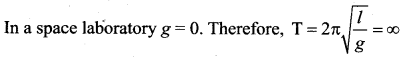

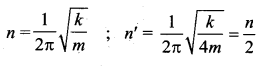



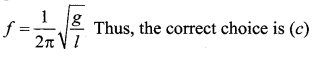
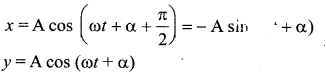
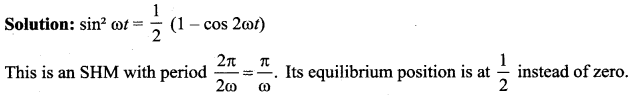
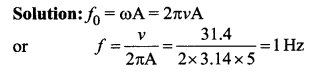

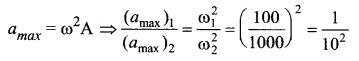
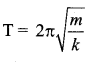
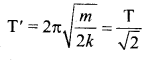
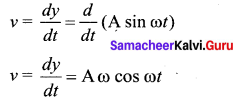
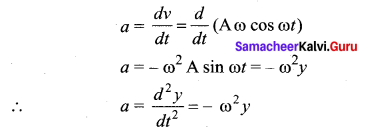
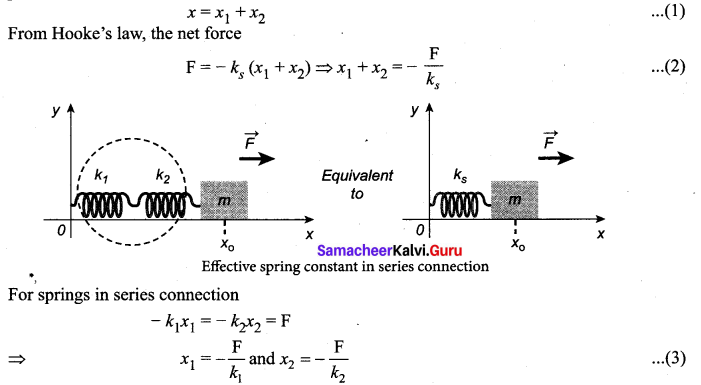

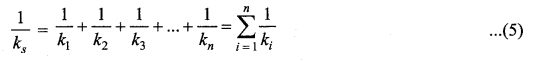

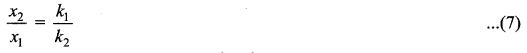

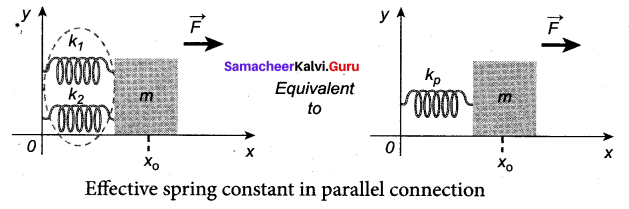

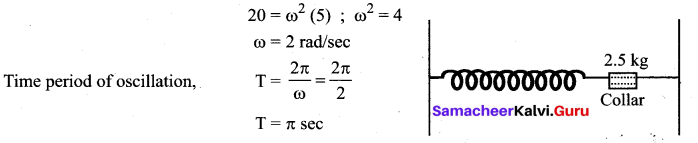
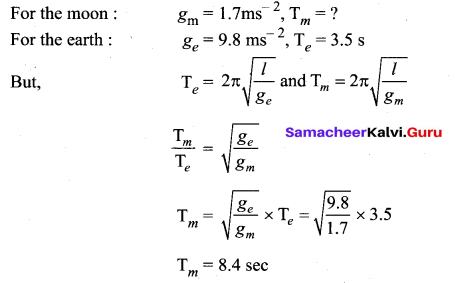
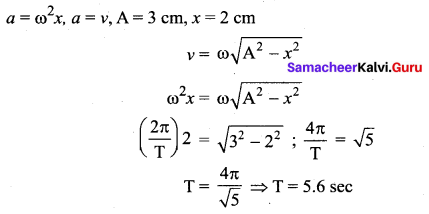
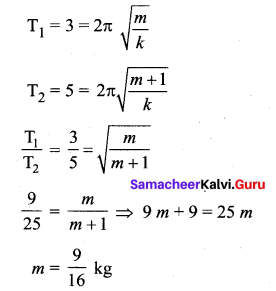
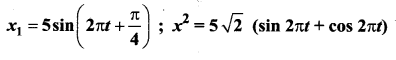

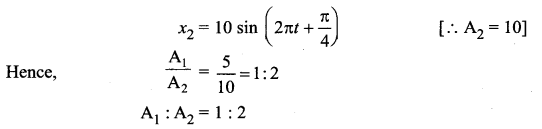
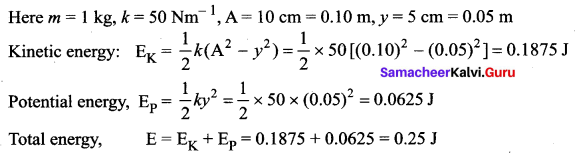
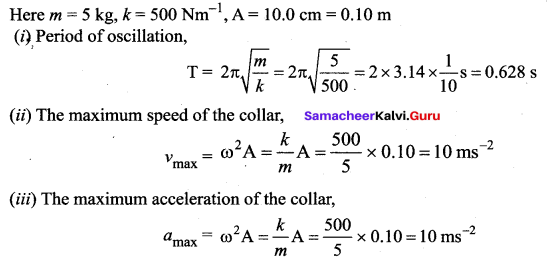
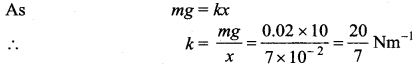
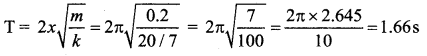
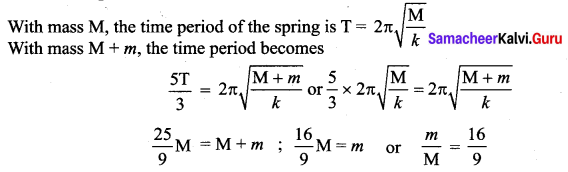
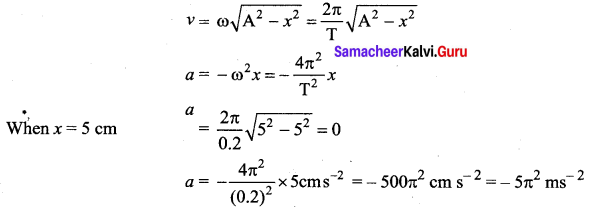
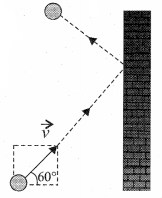


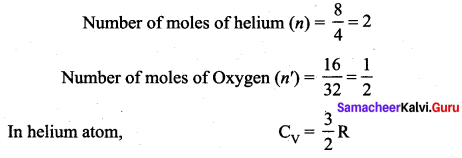
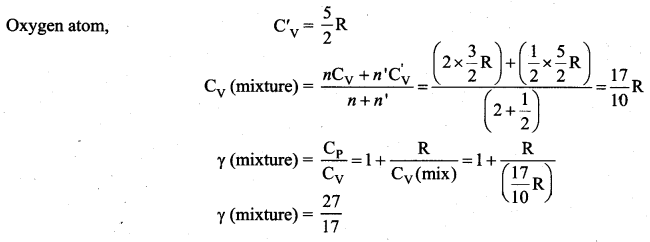

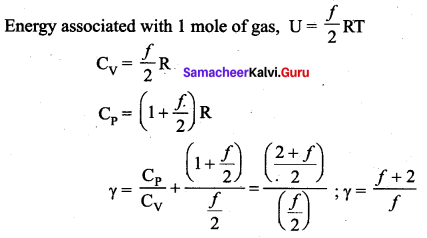
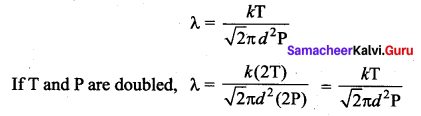
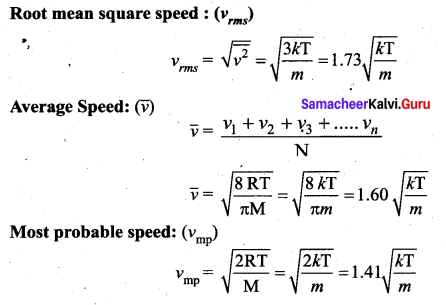



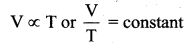
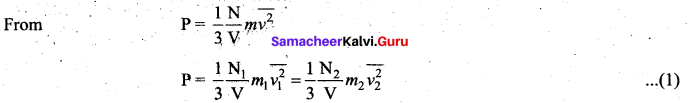



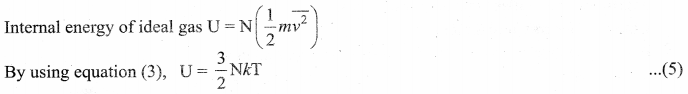
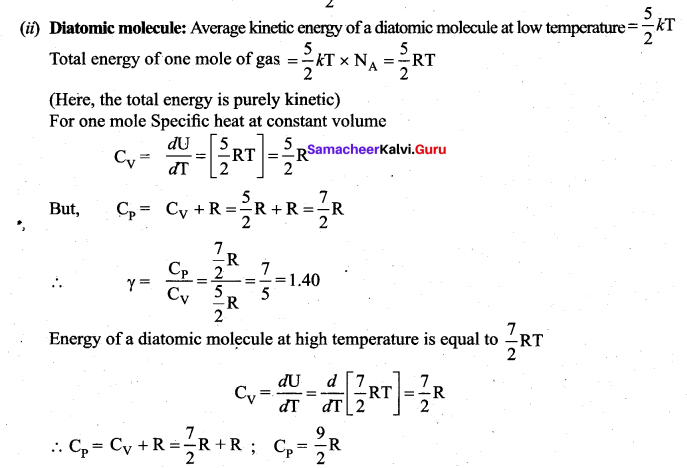
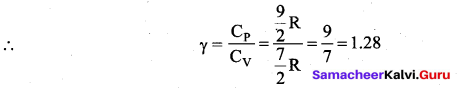
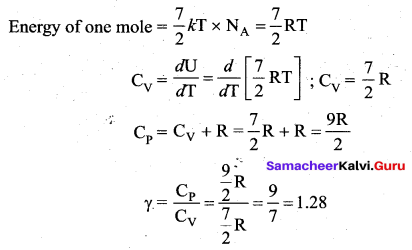
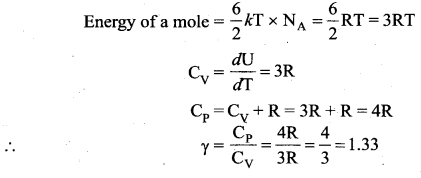
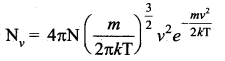
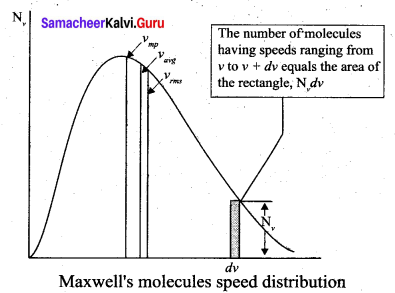
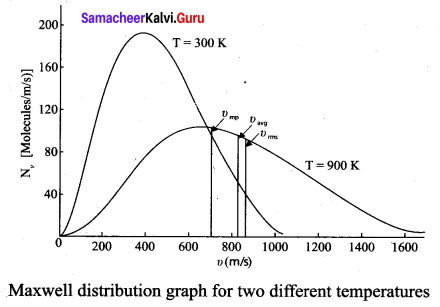

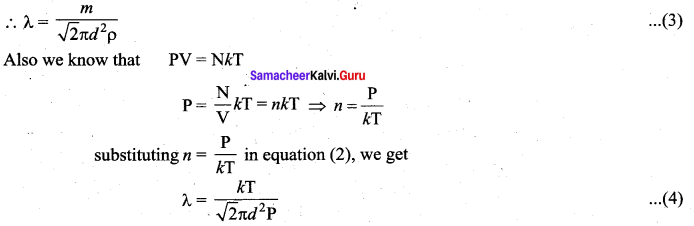
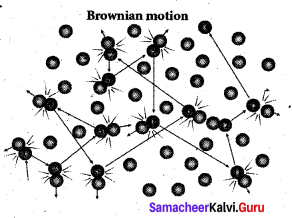
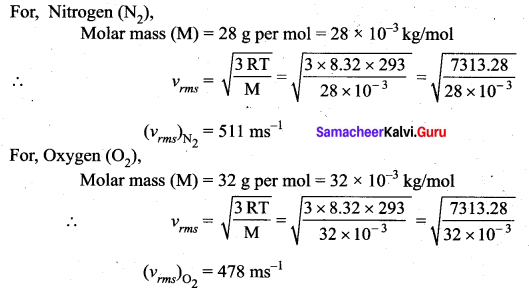
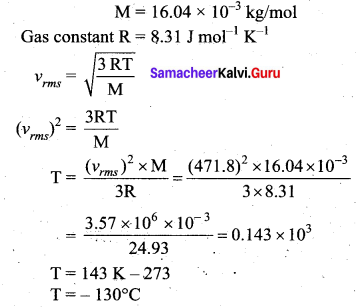
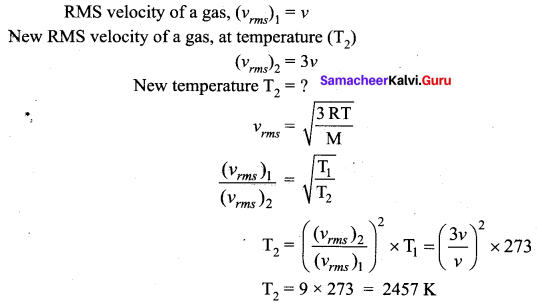
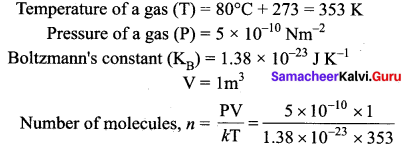
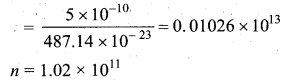
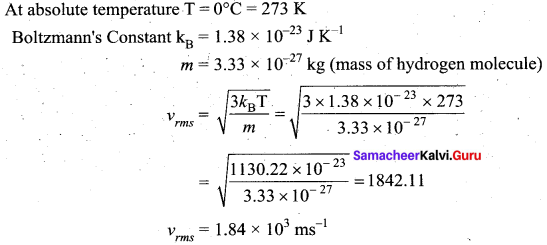
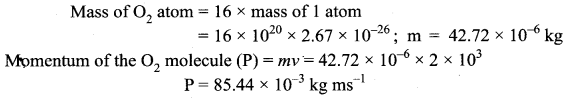

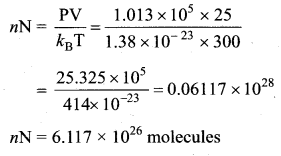
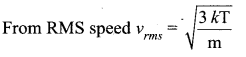
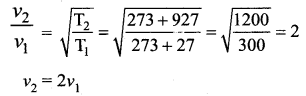
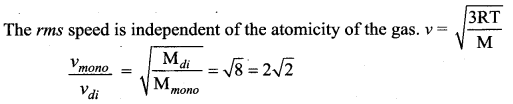
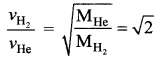


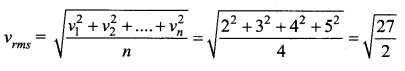

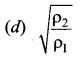
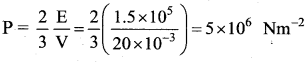
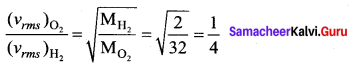
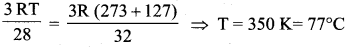 >
>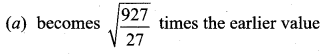
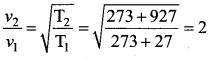
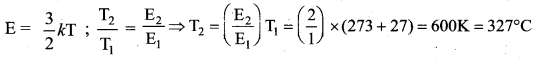
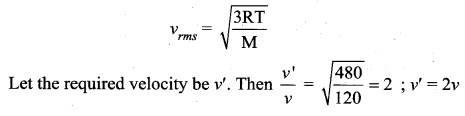

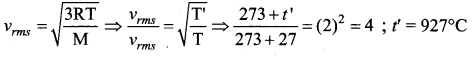

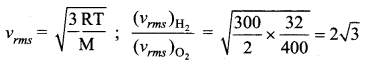
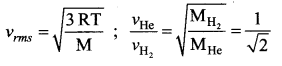
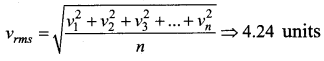
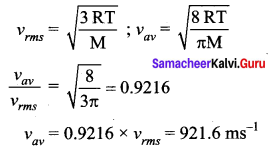
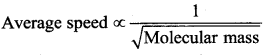
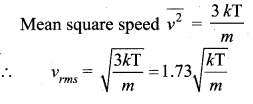
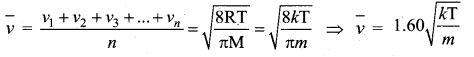
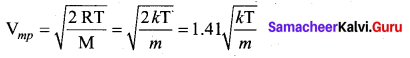

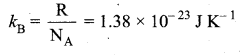
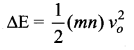
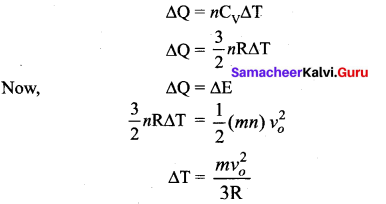


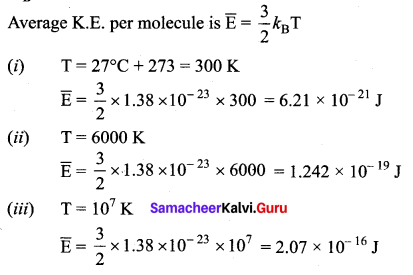
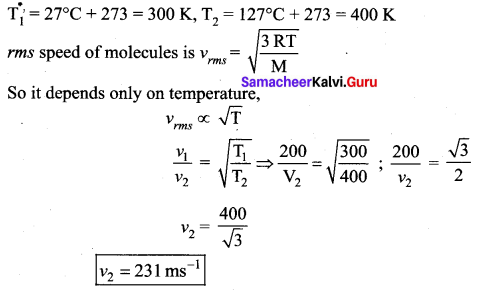

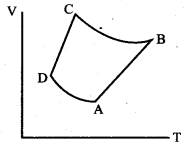
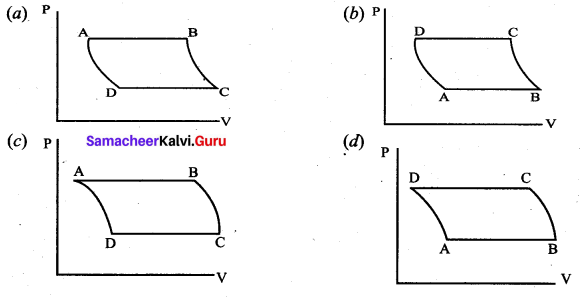
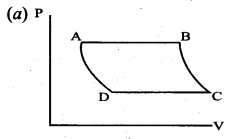
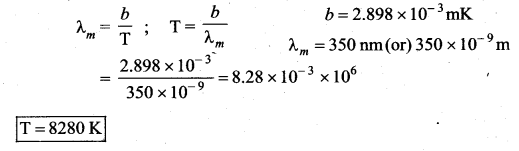
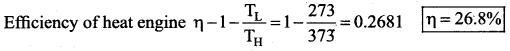
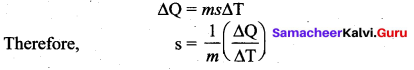
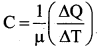
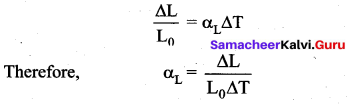
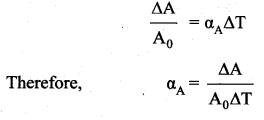
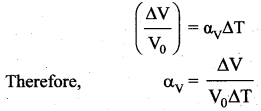
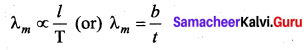
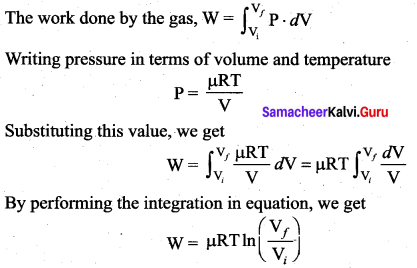
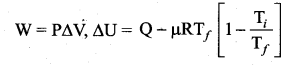
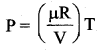

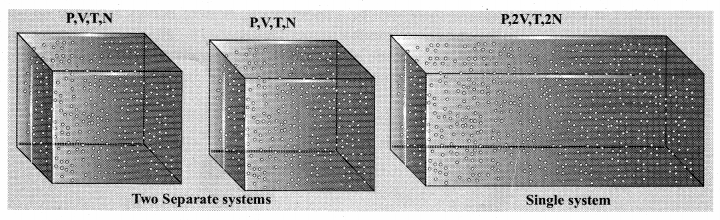
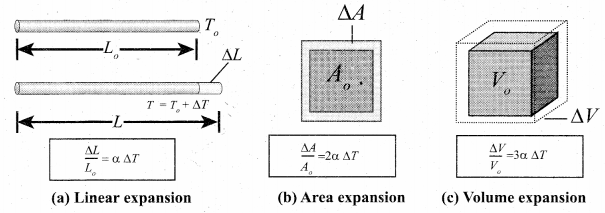
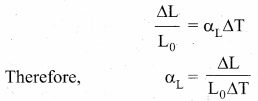
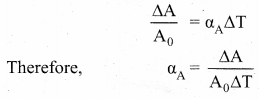
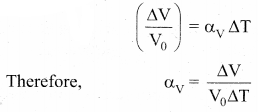
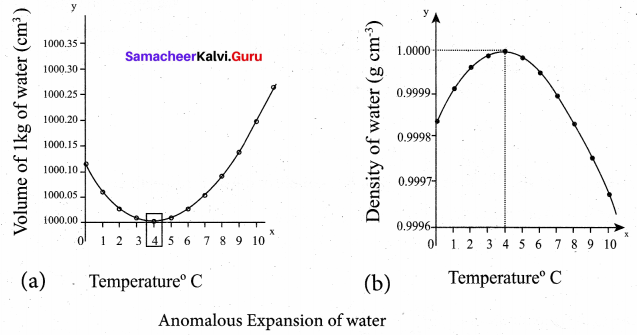
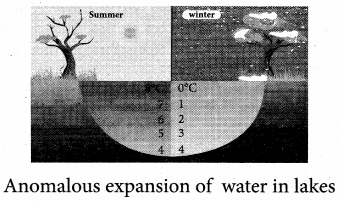
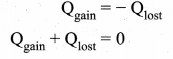
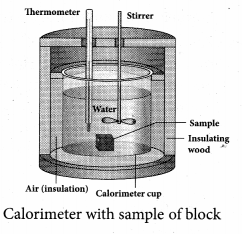
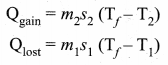
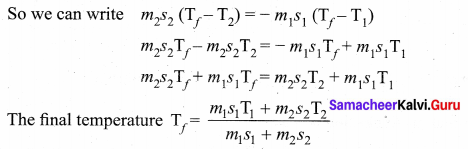
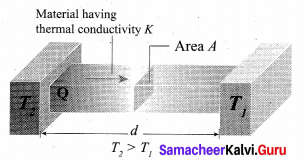
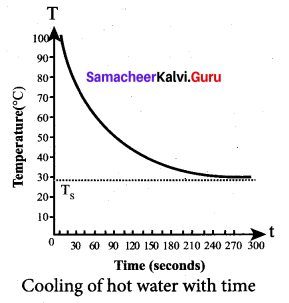
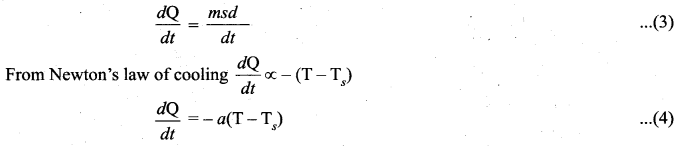
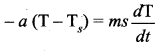

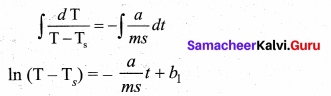

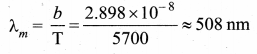
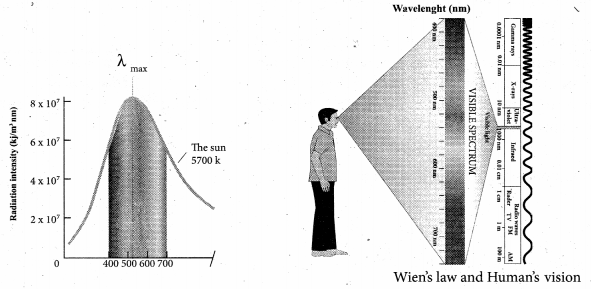
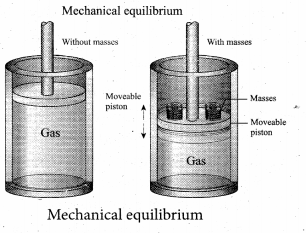
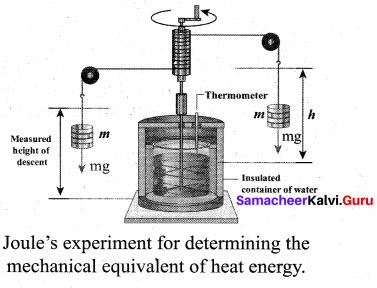
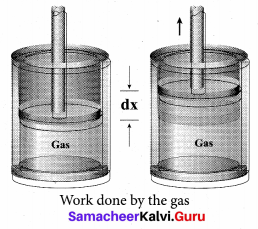

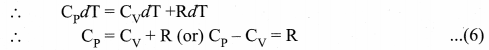
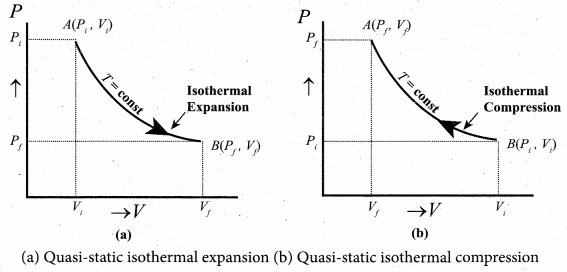


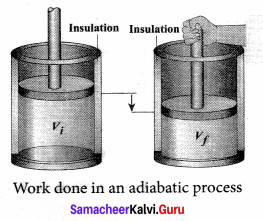
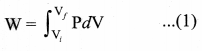
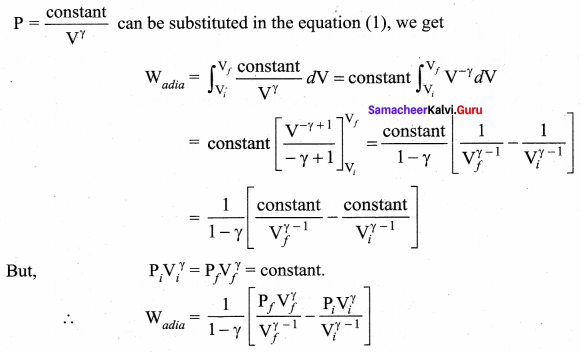
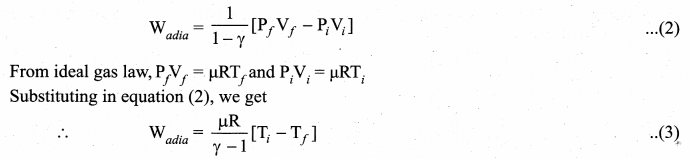
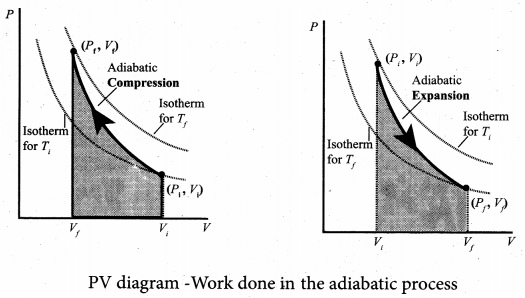
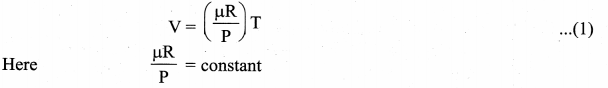

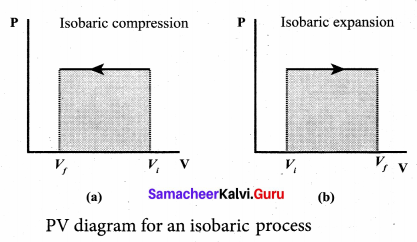

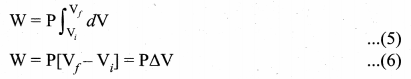
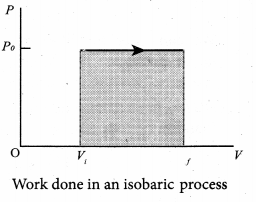

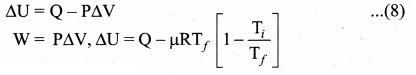

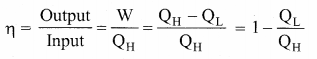
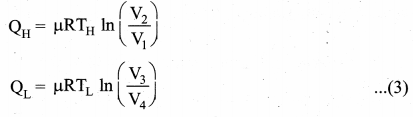

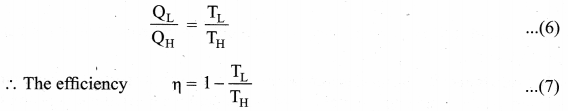

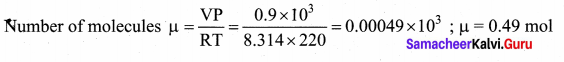
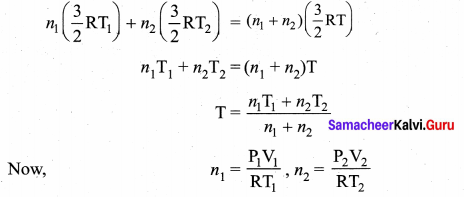
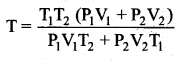
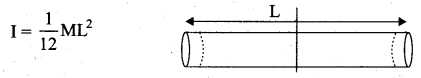
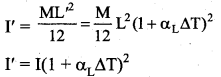
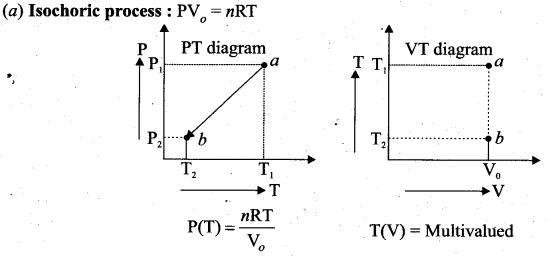
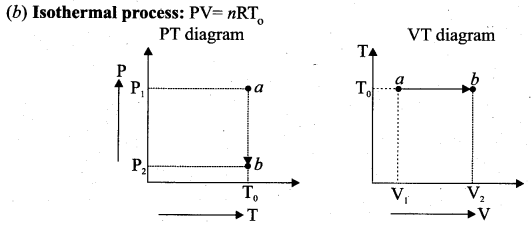
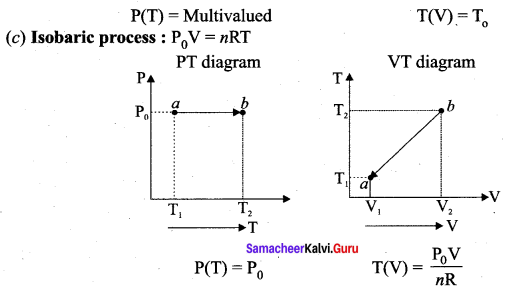
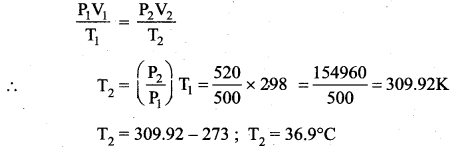
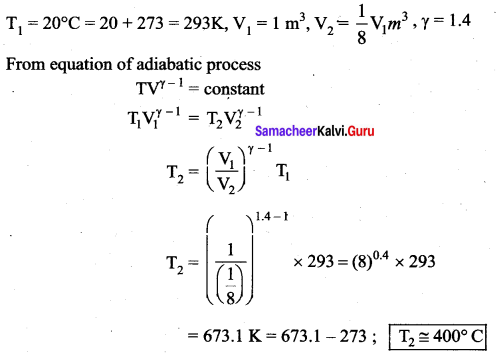
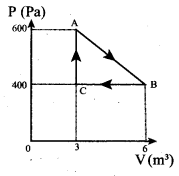
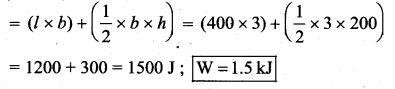
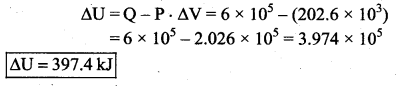
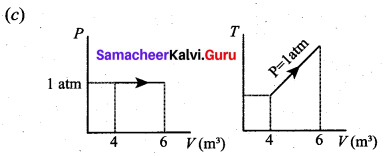
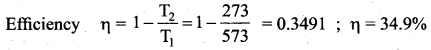
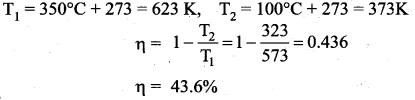
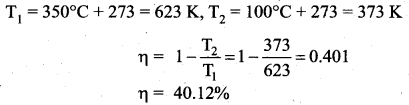
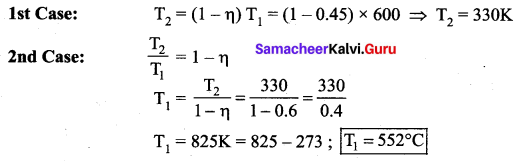

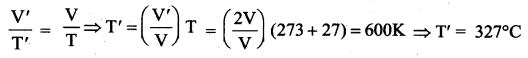
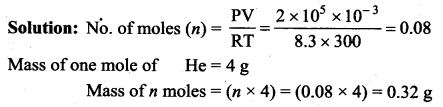
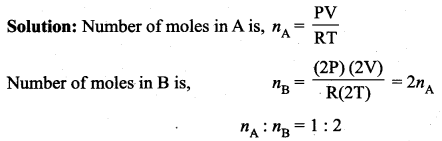
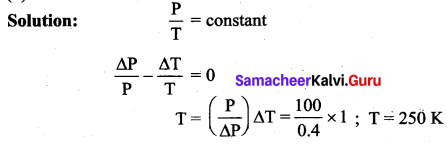
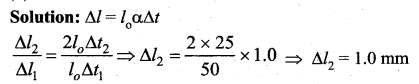
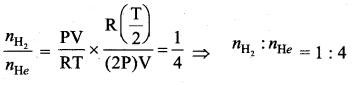
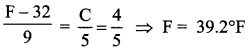
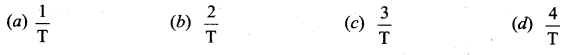
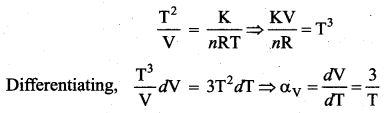
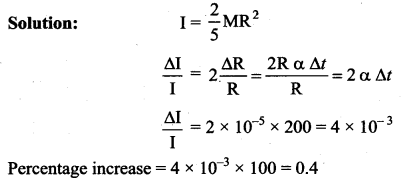
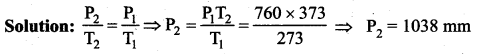
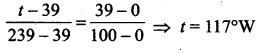
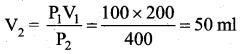
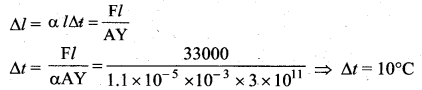
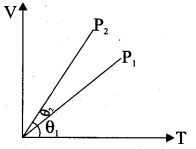


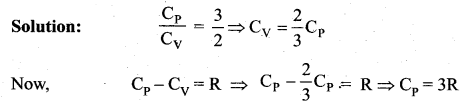
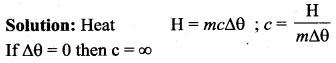


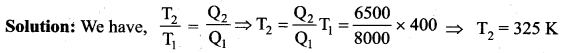
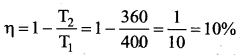
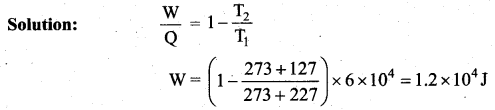
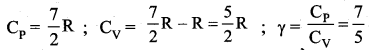
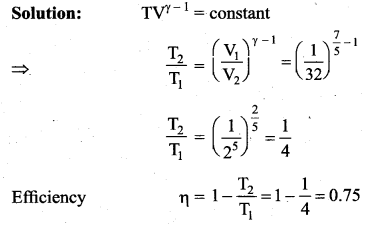
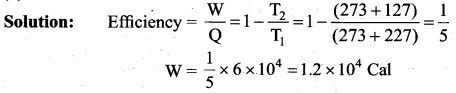
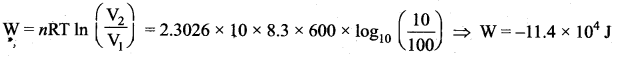
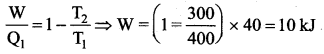
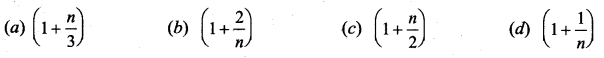
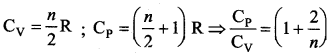
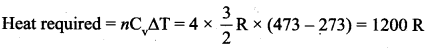
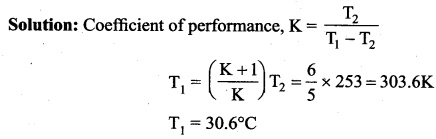
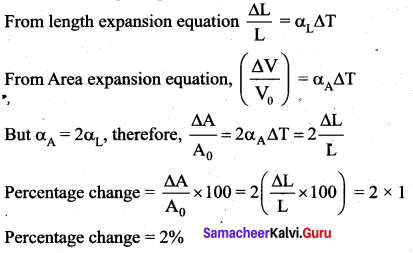
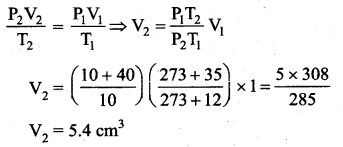
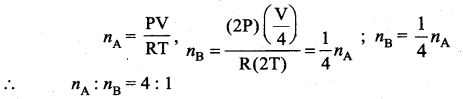
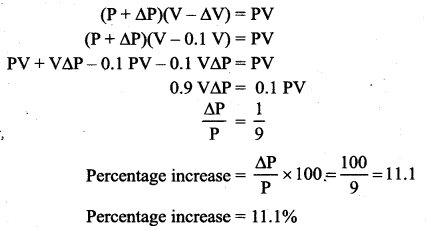
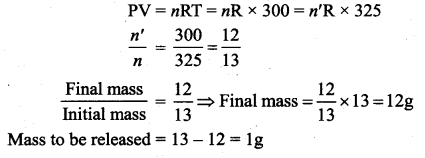

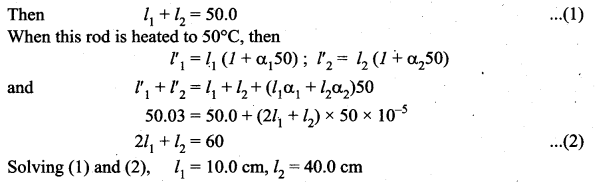

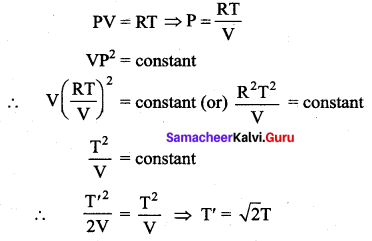
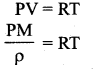

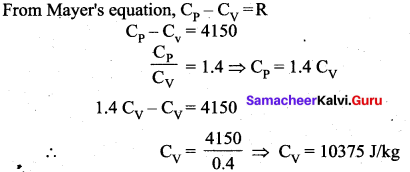
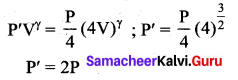

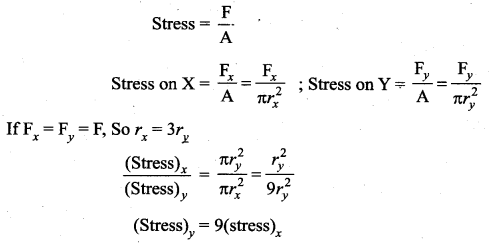

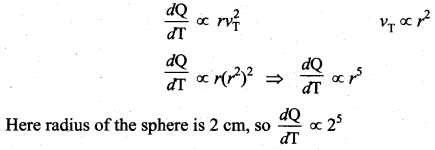
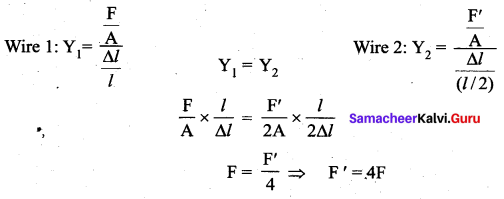
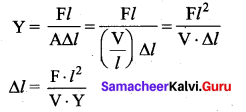
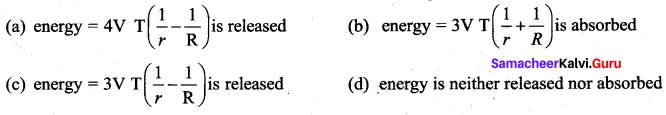
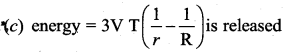
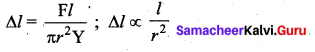
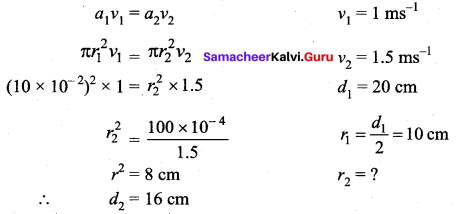
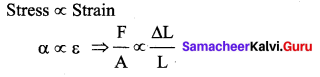



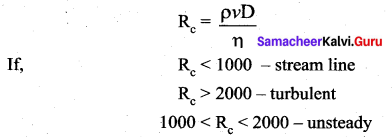
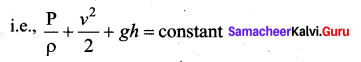
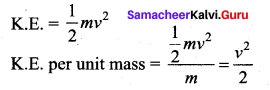
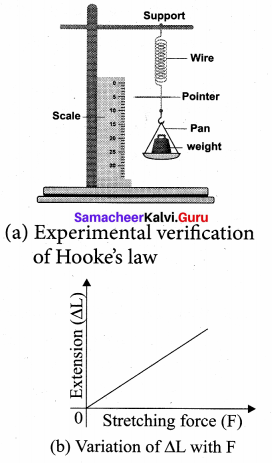
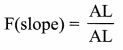
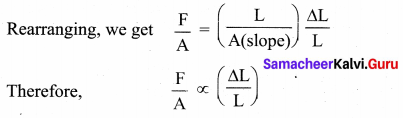
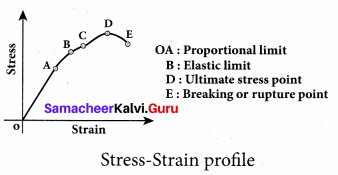
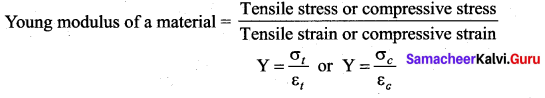
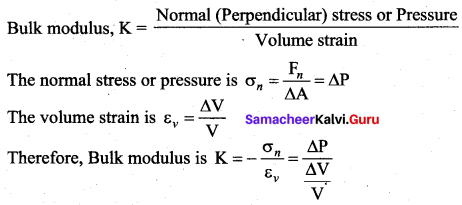
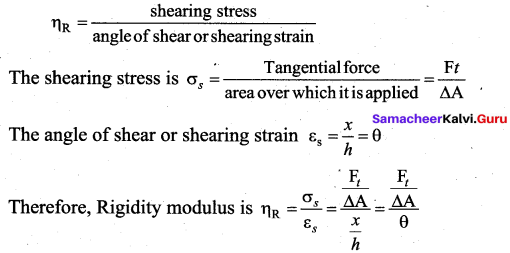
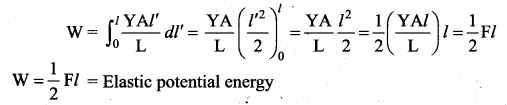

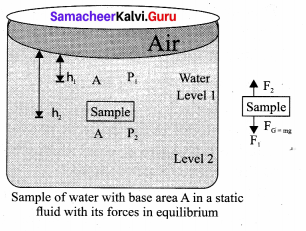
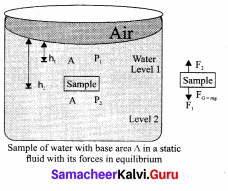
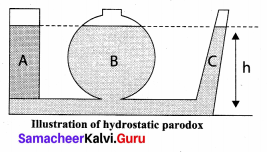
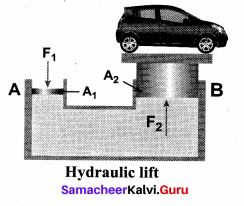
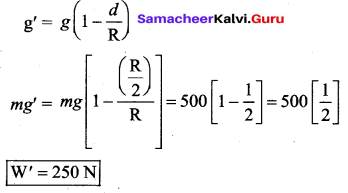
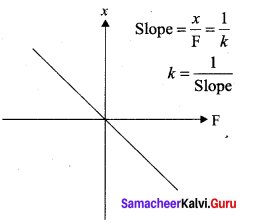
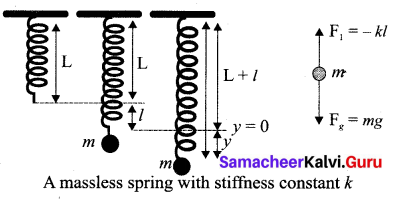
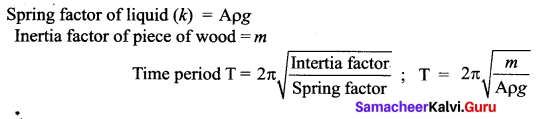
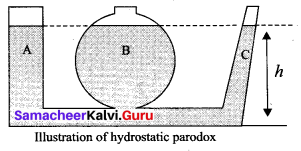
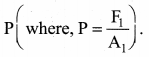 But according to Pascal’s law, this increased pressure P is transmitted undiminished in all directions. So a pressure is exerted on piston B. Upward force on piston B is
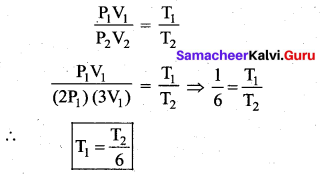
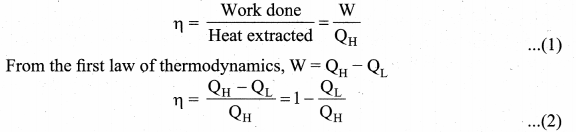
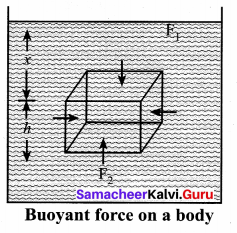
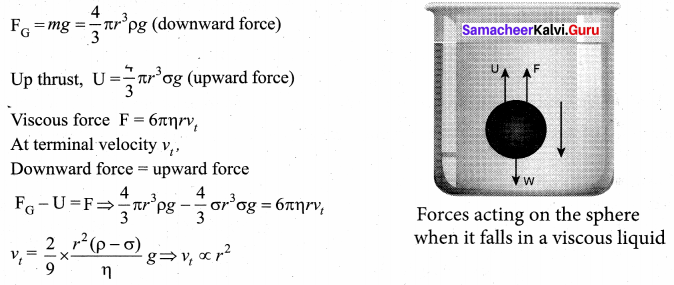
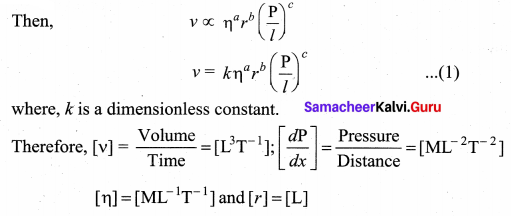
But according to Pascal’s law, this increased pressure P is transmitted undiminished in all directions. So a pressure is exerted on piston B. Upward force on piston B is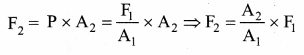
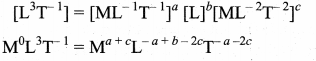
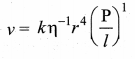

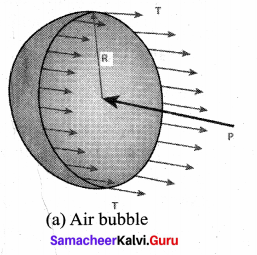
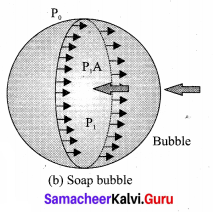

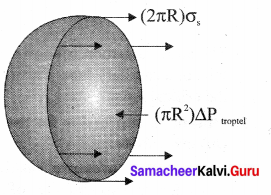

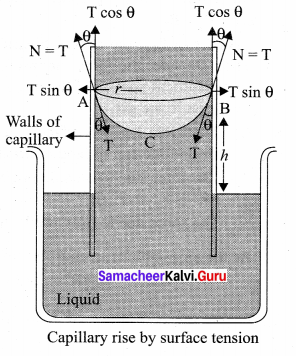
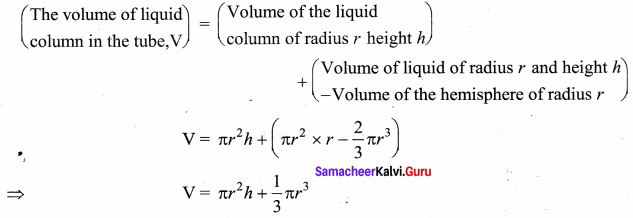
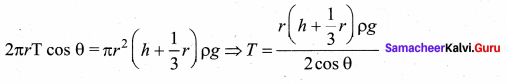
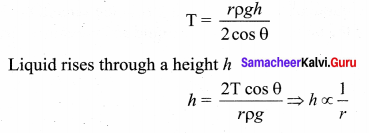
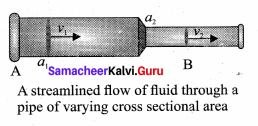
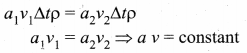
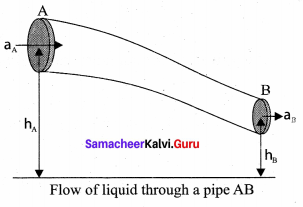
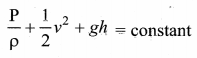
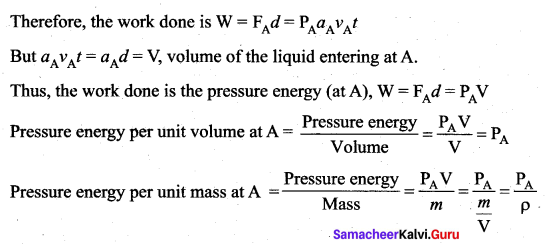
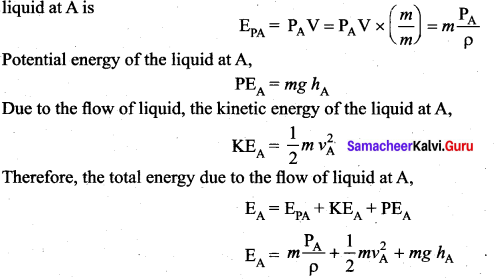
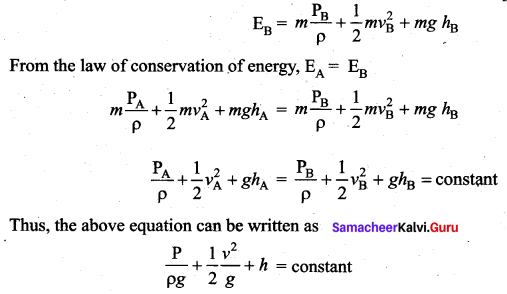
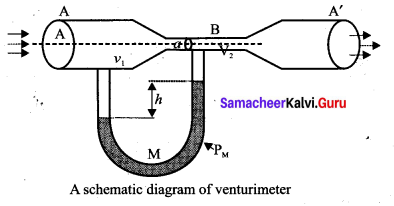
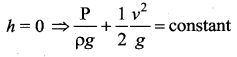
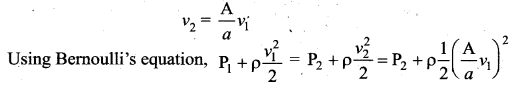
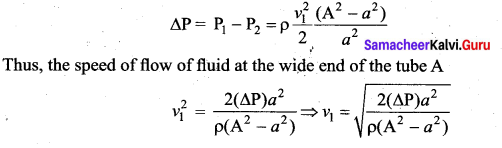
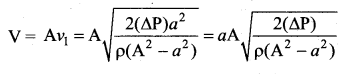
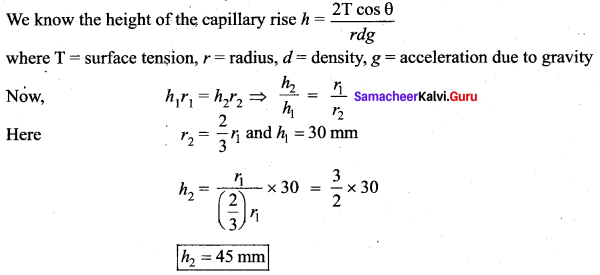
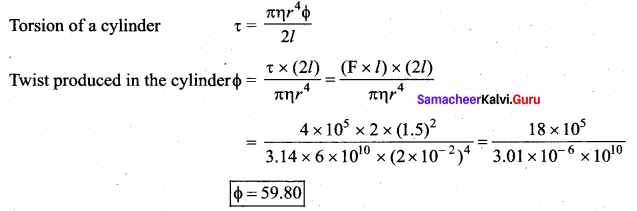

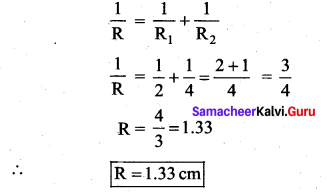
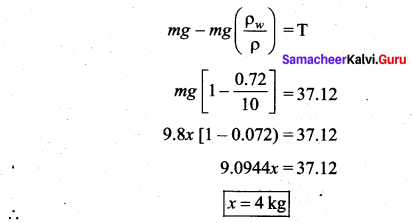
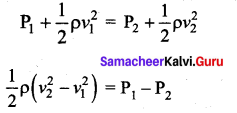
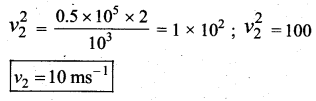
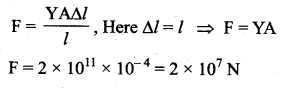
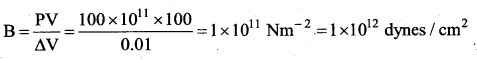
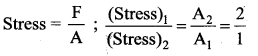
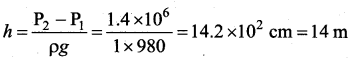
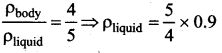
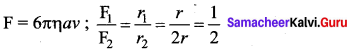
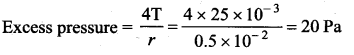
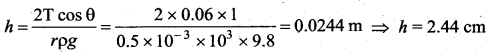
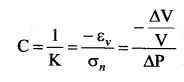
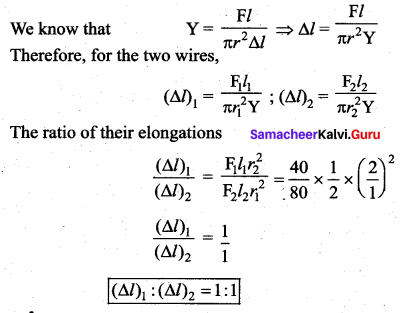
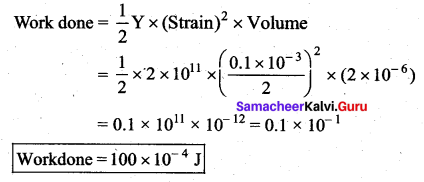
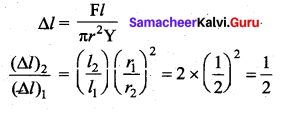
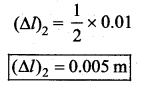
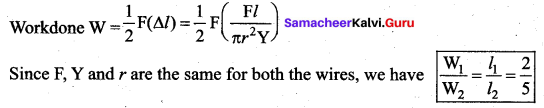
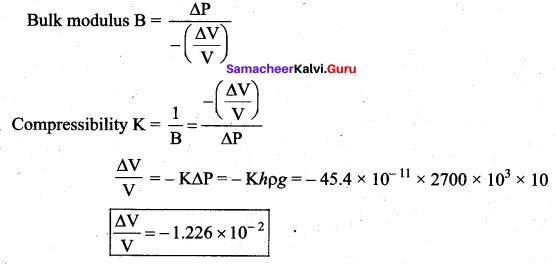
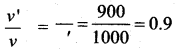
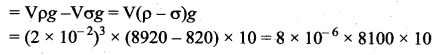
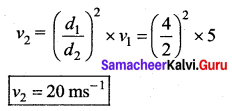
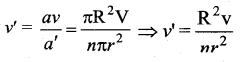
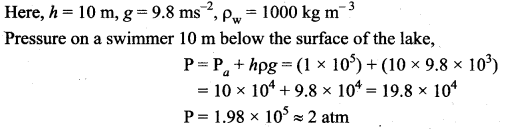
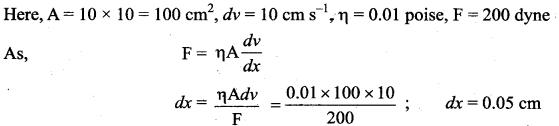
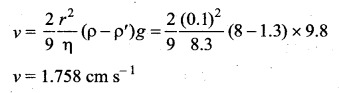
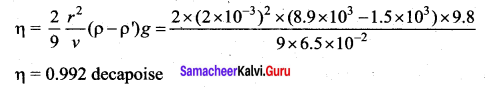
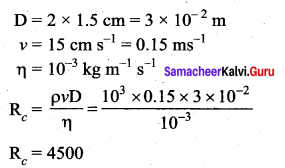
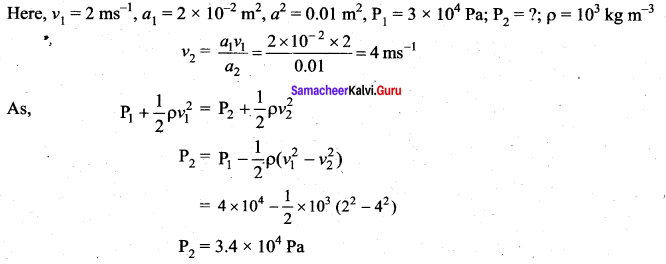
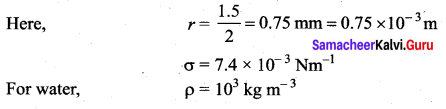
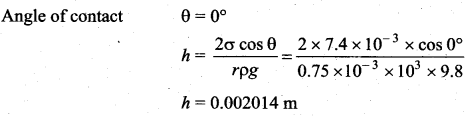
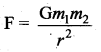
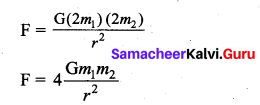
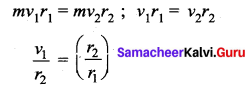
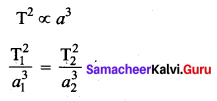
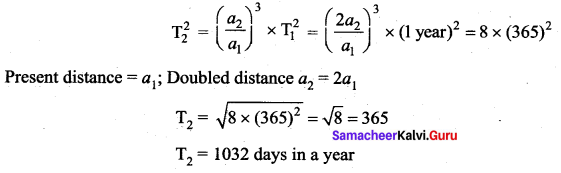

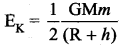
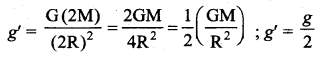
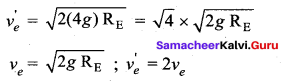
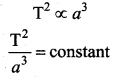
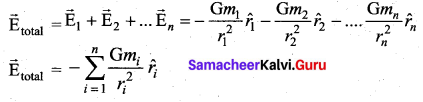
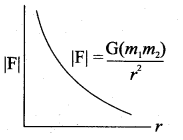
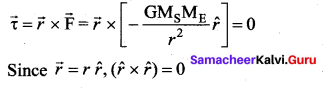
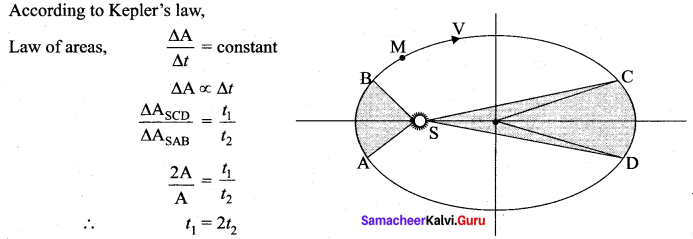
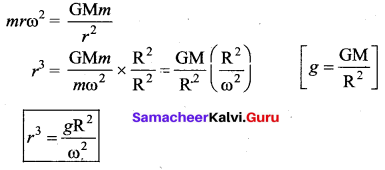
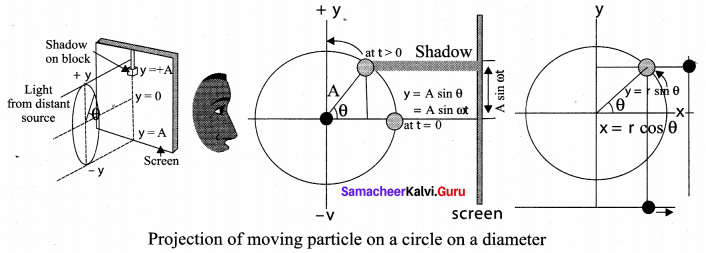
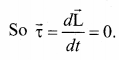 . It implies that angular momentum \(\overrightarrow{\mathrm{L}}\) is a constant vector.
. It implies that angular momentum \(\overrightarrow{\mathrm{L}}\) is a constant vector.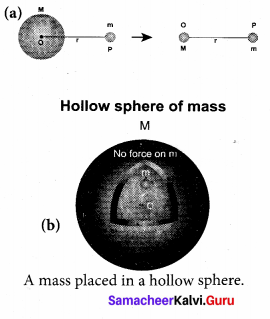
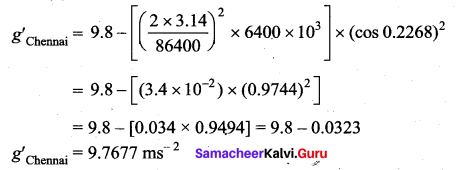
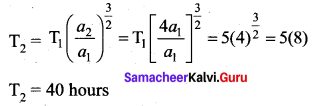
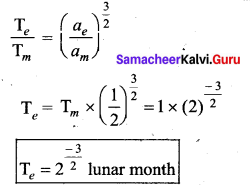
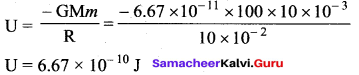
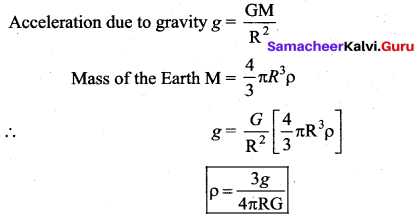
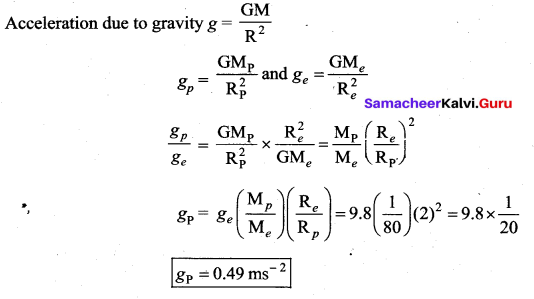
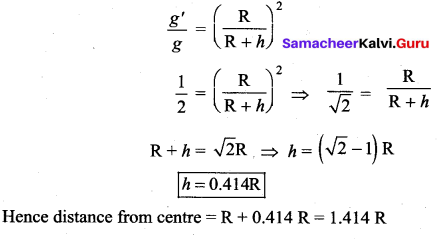
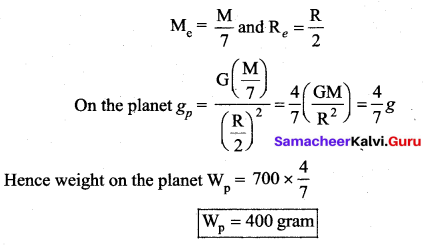
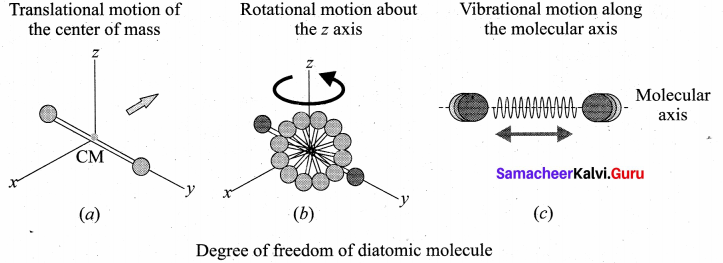
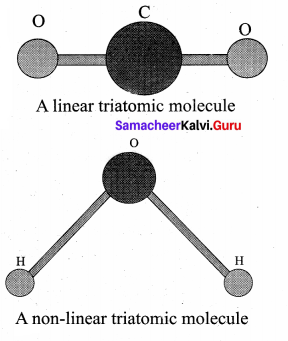
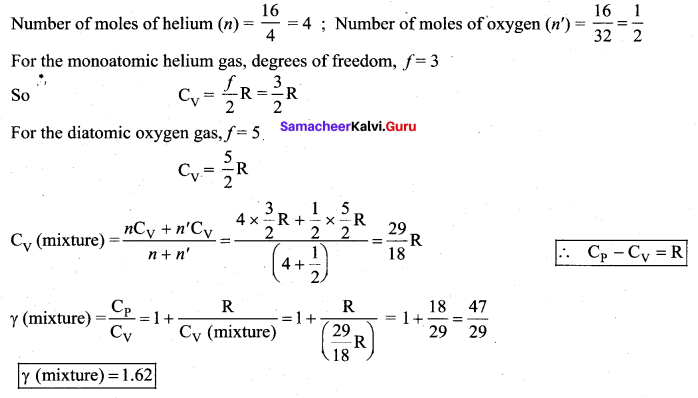
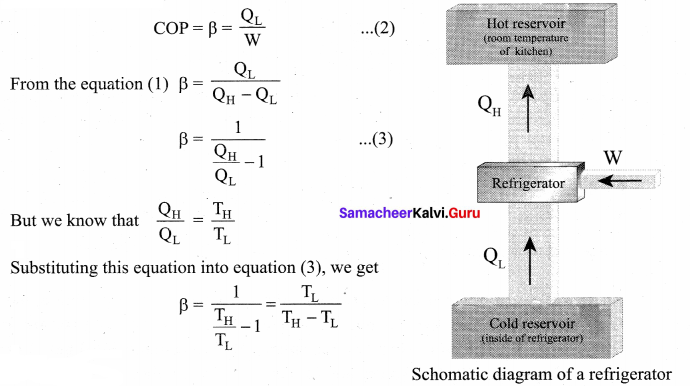
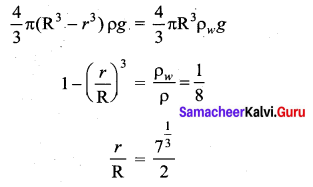
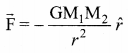 has one inherent assumption that both M1, and M2 are treated as point masses. When it is said that Earth orbits around the Sun due to Sun’s gravitational force, we assumed Earth and Sun to be point masses.
has one inherent assumption that both M1, and M2 are treated as point masses. When it is said that Earth orbits around the Sun due to Sun’s gravitational force, we assumed Earth and Sun to be point masses.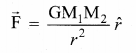


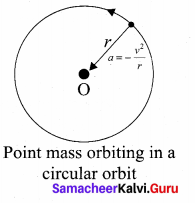


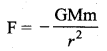


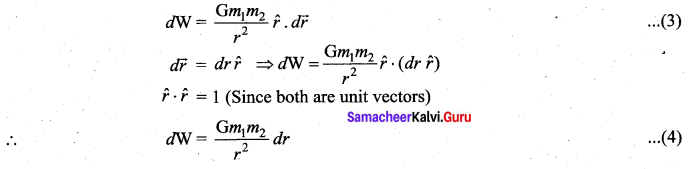
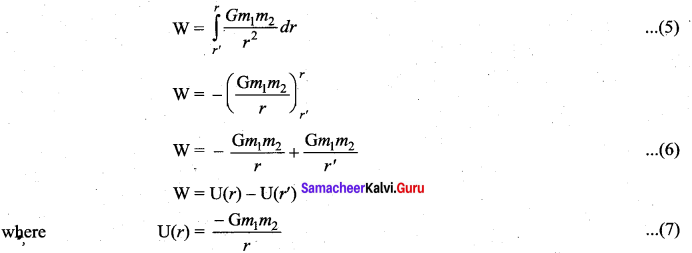
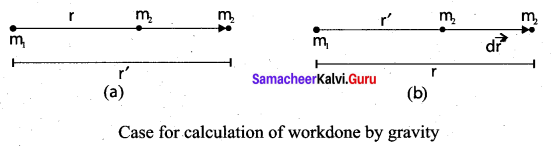
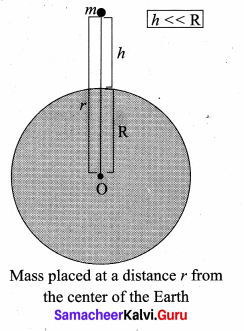

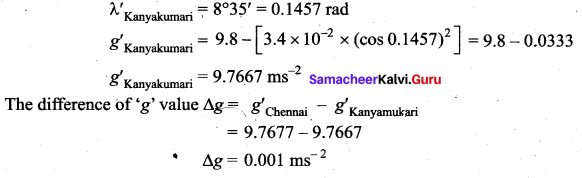
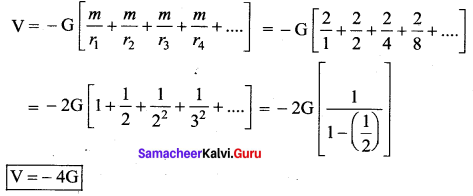
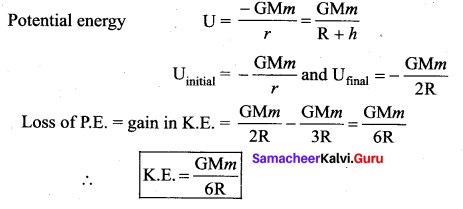
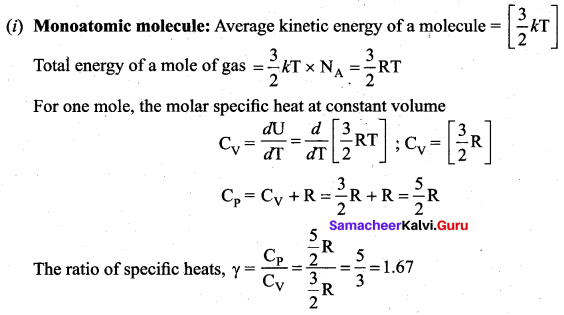
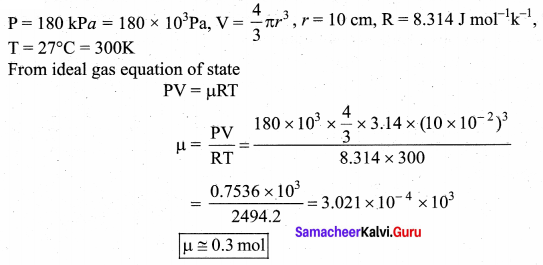
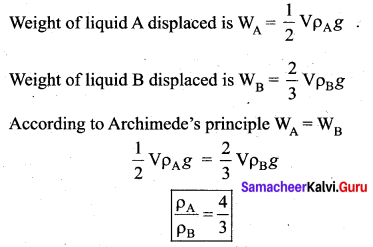
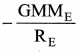 is the potential energy of the mass M.
is the potential energy of the mass M.
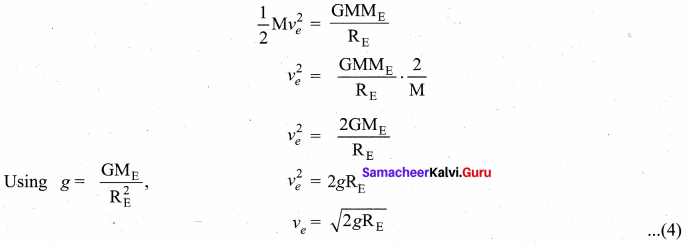
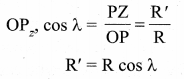
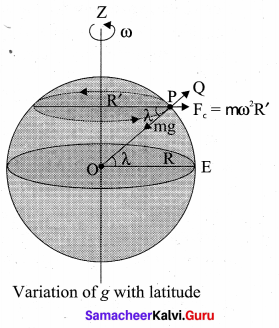
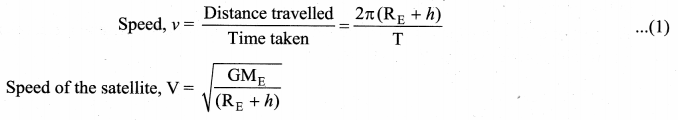


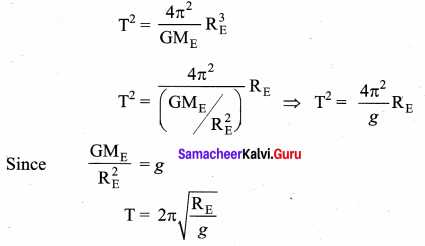

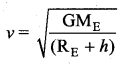
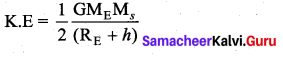

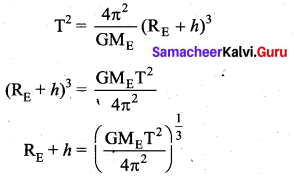
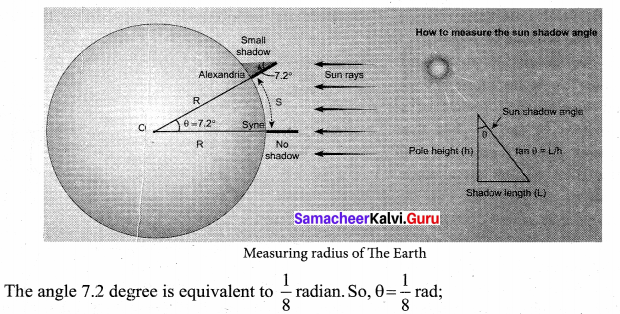

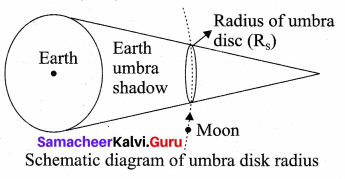
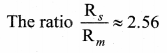



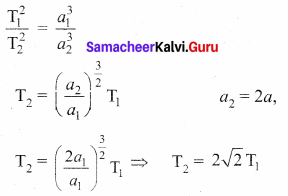
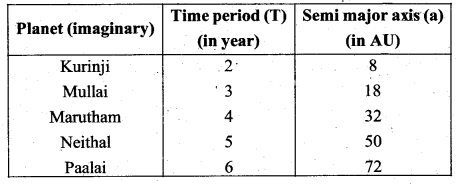

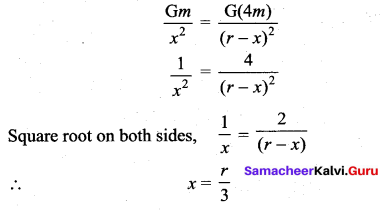
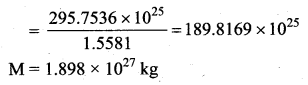
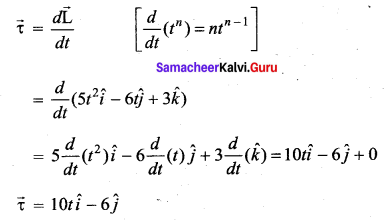
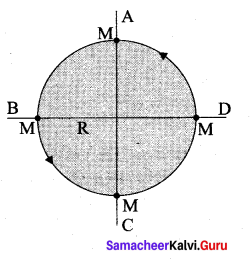
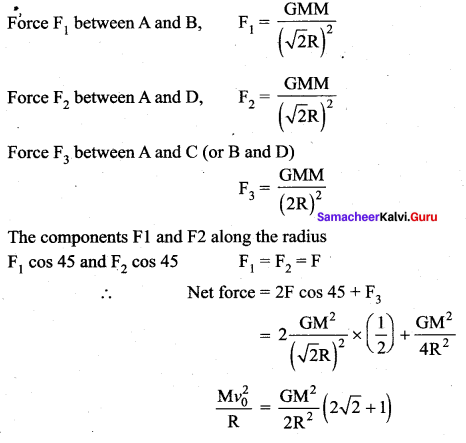
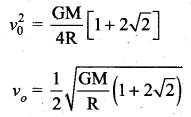
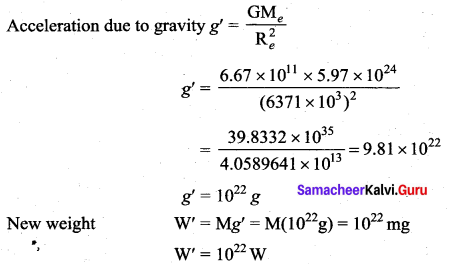

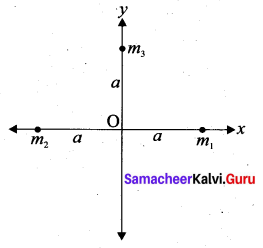
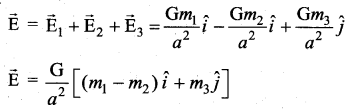
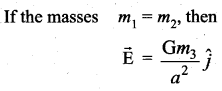

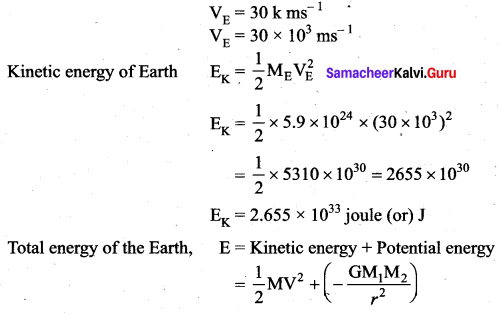
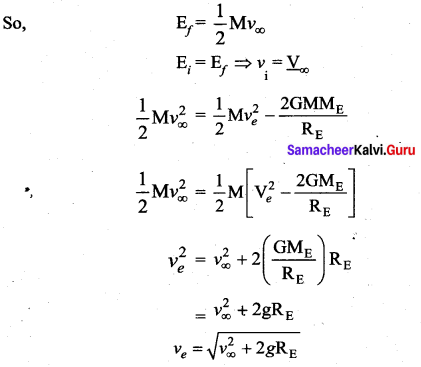
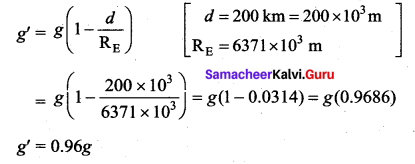
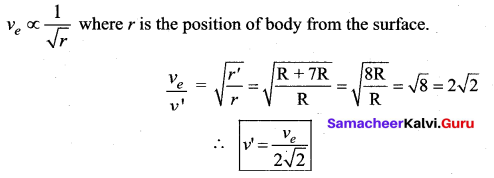
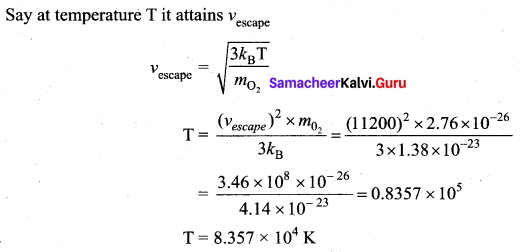
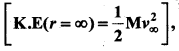 with that velocity should the object be thrown from Earth?
with that velocity should the object be thrown from Earth?