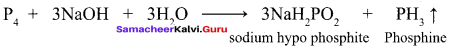
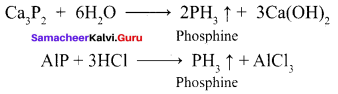
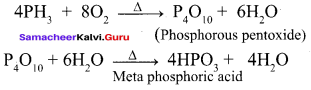
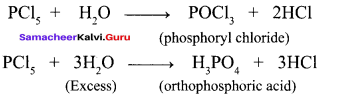
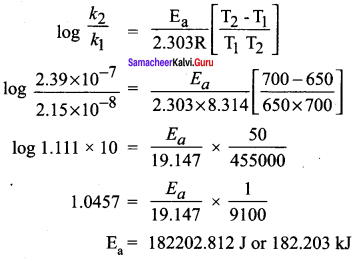
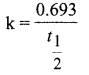Students can Download English Poem 4 Ulysses Questions and Answers, Summary, Activity, Notes, Samacheer Kalvi 12th English Book Solutions Guide Pdf helps you to revise the complete Tamilnadu State Board New Syllabus and score more marks in your examinations.
Tamilnadu Samacheer Kalvi 12th English Solutions Poem Chapter 4 Ulysses
12th English Poem Ulysses Warm Up
Introduction:
The poem ‘Ulysses’ is a dramatic monologue that contains 70 lines of blank verse. Ulysses, the king of Ithaca, gathers his men together to prepare for the journey and exhorts them not to waste their time left on earth. Ulysses has grown old, having experienced many adventures at the battle of Troy and in the seas. After returning to Ithaca, he desires to embark upon his next voyage. His inquisitive spirit is always looking forward to more and more of such adventures.

The poem can be divided into three parts:
- the thirst for adventure, which does not allow Ulysses to remain in his kingdom as a mere ruler
- Ulysses handing over the responsibility to his son Telemachus, with total confidence in his abilities
- Ulysses’ clarion call to his sailors, urging them to venture into unknown lands.
Samacheer Kalvi 12th Ulysses English Textual Questions
1. Complete the summary of the poem, choosing words from the list given below. Lines 1 to 32
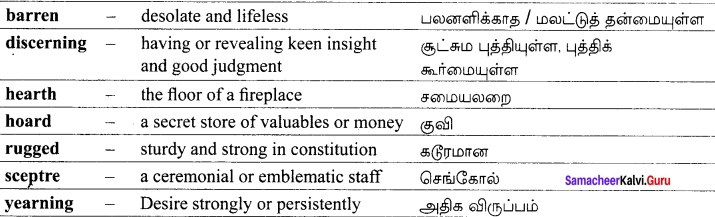
It little profits that an idle king,
By this still hearth, among these barren crags,
Match’d with an aged wife, I mete and dole
Unequal laws unto a savage race,
That hoard, and sleep, and feed, and know not me.
I cannot rest from travel: I will drink
Life to the lees: All times I have enjoy’d
Greatly, have suffer’d greatly, both with those
That loved me, and alone, on shore, and when
Thro’ scudding drifts the rainy Hyades
Vext the dim sea: I am become a name;
For always roaming with a hungry heart
Much have I seen and known; cities of men
And manners, climates, councils, governments,
Myself not least, but honour’d of them all;
And drunk delight of battle with my peers,
Far on the ringing plains of windy Troy.
I am a part of all that I have met;
Yet all experience is an arch wherethro’
Gleams that untravell’d world whose margin fades
For ever and forever when I move.
How dull it is to pause, to make an end,
To rust unburnish’d, not to shine in use!
As tho’ to breathe were life! Life piled on life
Were all too little, and of one to me
Little remains: but every hour is saved
From that eternal silence, something more,
A bringer of new things; and vile it were
For some three suns to store and hoard myself,
And this gray spirit yearning in desire
To follow knowledge like a sinking star,
Beyond the utmost bound of human thought.
This is my son, mine own Telemachus,
To whom I leave the sceptre and the isle, –
Well-loved of me, discerning to fulfil
This labour, by slow prudence to make mild
A rugged people, and thro’ soft degrees
Subdue them to the useful and the good.
Most blameless is he, centred in the sphere
Of common duties, decent not to fail
offices of tenderness, and pay
Meet adoration to my household gods,
When I am gone. He works his work, I mine.
There lies the port; the vessel puffs her sail:

There gloom the dark, broad seas. My mariners,
Souls that have toil’d, and wrought, and thought with
me That ever with a frolic welcome took
The thunder and the sunshine, and opposed
Free hearts, free foreheads – you and I are old;
Old age hath yet his honour and his toil;
Death closes all: but something ere the end,
Some work of noble note, may yet be done,
Not unbecoming men that strove with Gods.
The lights begin to twinkle from the rocks:
The long day wanes: the slow moon climbs: the deep
Moans round with many voices. Come, my friends,
‘T is not too late to seek a newer world.
Push off, and sitting well in order smite
The sounding furrows; for my purpose holds
To sail beyond the sunset, and the baths
Of all the western stars, until I die.
It may be that the gulfs will wash us down:
It may be we shall touch the Happy Isles,
And see the great Achilles, whom we knew.
Tho’ much is taken, much abides; and tho’
We are not now that strength which in old days
Moved earth and heaven, that which we are, we are;
One equal temper of heroic hearts,
Made weak by time and fate, but strong in will
To strive, to seek, to find, and not to yield.

| fullest | unquenchable | unattainable |
| experience | knowledge | king |
| matters | rust | adventure |
| unwilling | travel | breathing |
Ulysses is (1) ______ to discharge his duties as a (2) ______ , as he longs for (3) ______ He is filled with an (4) ______ thirst for (5) ______ and wishes to live life to the (6) ______ He has travelled far and wide gaining (7) ______ of various places, cultures, men and (8) ______ He recalls with delight his experience at the battle of Troy. Enriched by his (9) ______ he longs for more and his quest seems endless. Like metal which would (10) ______ if unused, life without adventure is meaningless. According to him living is not merely (11) ______ to stay alive. Though old but zestful, Ulysses looks at every hour as a bringer of new things and yearns to follow knowledge even if it is (12) ______
Answers:
- unwilling
- king
- adventure
- unquenchable
- travel
- fullest
- experience
- matters
- knowledge
- rust
- breathing
- unattainable
Lines 33 to 42
| prudence | kingdom | quest | tender |
Ulysses desires to hand over his (1) _____ to his son Telemachus, who would fulfil his duties towards his subjects with care and (2) _____ Telemachus possesses patience and has the will to civilise the citizens of Ithaca in a (3) _____ way. Ulysses is happy that his son would do his work blamelessly and he would pursue his (4) _____ for travel and knowledge.
Answer:
- kingdom
- prudence
- tender
- quest
Lines 44 to 70
| world | thunder | meaningful |
| gather | undaunted | heaven |
Ulysses beckons his sailors to (1) _____ at the port where the ship is ready to sail. His companions who have faced both (2) _____ and sunshine with a smile, are united by their undying spirit of adventure. Though death would end everything, Ulysses urges his companions to join him and sail beyond the sunset and seek a newer (3) _____ , regardless of consequences. These brave hearts who had once moved (4) _____ and earth, may have grown old and weak . physically but their spirit is young and (5) _____ His call is an inspiration for all those who seek true knowledge and strive to lead (6) _____ lives.
Answers:
- gather
- thunder
- world
- heaven
- undaunted
- meaningful
2. Answer the following questions in one or two sentences each.
Ulysses Poem Questions And Answers Question (а)
‘Ulysses is not happy to perform his duties as a king.’ Why?
Answer:
Ulysses is not happy to perform the ordinary duties of a king mainly because his heart is in voyages beyond horizon. He is bored with the task of enforcing law and order and giving reward and punishment to a savage race.
Ulysses Questions And Answers Question (b)
What does he think of the people of his kingdom?
Answer:
The citizen of Ithaca hoard, sleep and feed. They don’t understand the aspirations of the dreamer Ulysses.
12th English Poem Ulysses Paragraph Question (c)
What has Ulysses gained from his travel experiences?
Answer:
Ulysses has met people hailing from different cultural backgrounds. He has learnt much from their manners, climates, councils and governments. He learnt strategies of warfare in battles.
Ulysses Poem Erc Question (d)
Pick out the lines which convey that his quest for travel is unending.
Answer:
“How dull it is to pause, to make an end,
To rust unburnished, not to shine in use!
The lines quoted above convey his quest for travel is unending.
12th English Ulysses Paragraph Question (e)
‘As tho’ to breathe were life!’ – From the given line what do you understand of Ulysses’ attitude to life?
Answer:
Ulysses strongly believes that just breathing is not life. Life has to be adventurous and full of action.
Ulysses Poem Questions And Answers Pdf Question (J)
What does Ulysses yearn for?
Answer:
Ulysses yearns for following knowledge like a sinking star beyond the utmost bound of human thought.
Ulysses Question Answer Question (g)
Who does the speaker address in the second part?
Answer:
The speaker addresses the readers in the second part explaining the difference between his roles and that of Telemachus.
Ulysses Poem Short Questions And Answers Question (h)
Why did Ulysses want to hand over the kingdom to his son?
Answer:
Ulysses finds Telemachus discerning and prudent. Besides, Ulysses is wearing the crown uneasily as the call for adventure and desire to sail beyond sunset is obsessing his mind. So, he wants to hand over the kingdom to Telemachus.
Ulysses Short Questions And Answers Question (i)
How would Telemachus transform the subjects?
Answer:
Ulysses believes that his son Telemachus is wise and kind enough to transform rugged citizens into mild and civilized subjects by his tenderness and love.
Question (j)
‘He works his work, I mine’ – How is the work distinguished?
Answer:
Telemachus would do the work of ruling Ithaca with prudence and tenderness. Ulysses will pursue his dream of adventure and try to meet great Achilles in the other world.
Question (i)
In what ways were Ulysses and his mariners alike?
Answer:
Both Ulysses and his fellow sailors are now old. They no more have the strength they possessed in olden days moving earth and heaven. They are made weak by time and fate but strong in will “to strive, to seek, to find and not to yield.” They share the heroic temper and undying quest for knowledge and adventure.
Question (j)
What could be the possible outcomes of their travel?
Answer:
The sailors and Ulysses may be washed down by the gulfs or they could touch Greek paradise and meet their hero Achilles. They may die happily braving the elements of nature.
3. Identify the figures of speech employed in the following lines.
Question (a)
“Thro” scudding drifts the rainy Hyades
Vext the dim sea…”
Answer:
The figure of speech Personification is employed in the above lines.
Question (b)
“For always roaming with a hungry heart”
Answer:
Metaphor
Question (c)
“And drunk delight of battle with my peers;”
Answer:
Metaphor
Question (d)
“…..the deep
Moans round with many voices.”
Answer:
Personification
Question (e)
“To follow knowledge like a sinking star.”
Answer:
Simile
Question (f)
“ There lies the port the vessel puffs her sai”
Answer:
Personification
Additional Questions
Question (a)
“I will drink life to the lees'”
Answer:
Metaphor
Question (b)
“Vext the dim sea:”
Answer:
Personification
Question (c)
“Yet all experience is an arch wherethro”
Answer:
Metaphor
Question (d)
“Gleams that untravell’d world whose margin fades”
Answer:
Metaphor
Question (e)
“To rust unburnish’d, not to shine in use!”
Answer:
Metaphor
Question (f)
“There gloom the dark, broad seas. My mariners,”
Answer:
Metaphor
Question (g)
“Souls that have toil’d, and wrought, and thought with me”
Answer:
Synecdoche (part of the whole)
Question (h)
“The thunder and the sunshine, and opposed”
Answer:
Metaphor
Question (i)
“T is not too late to seek a newer world.”
Answer:
Synecdoche
Question (j)
“…in order smite The sounding furrows;”
Answer:
Metaphor
Question (k)
“To sail beyond the sunset, and the baths”
Answer:
Metaphor
Question (l)
“It may be we shall touch the Happy Isles,”
Answer:
Allusion (in Greek mythology the place is known as Greek paradise)
Question (m)
“And see the great Achilles, whom we knew.”
Answer:
Allusion (Greek mythology)
Appreciate The Poem
4. Read the sets of lines from the poem and answer the questions that follow.
(a) “…I mete and dole
Unequal laws unto a savage race,
That hoard, and sleep, and feed, and know not me.”
Question (i)
What does Ulysses do?
Answer:
Ulysses, like a grocery shop owner, measures and delivers rewards and punishments unto a large number of uncivilized citizens.
Question (ii)
Did he enjoy what he was doing? Give reasons.
Answer:
No, he did not enjoy is work. He does not like the idea of ministering variable justice to people who like “drones” or animals just eat, sleep and multiply their kind. He wants to leave such work to his son.
Question (b)
“Yet all experience is an arch wherethrough
Gleams that untravelled world, whose margin fades
For ever and for ever when I move. ”
Question (i)
What is experience compared to?
Answer:
Experience is compared to an arch.
Question (ii)
How do the lines convey that the experience is endless?
Answer:
Through the arch of experience one can see the untravelled world. But the experience in the untravelled has a margin whose border fades as one moves forward. Thus experience is endless.
Question (c)
“Little remains: but every hour is saved
From that eternal silence, something more,
A bringer of new things; and vile it were”
Question (i)
How is every hour important to Ulysses?
Answer:
One lives in this world for a limited time. Every hour can provide new knowledge. So, every hour is very important.
Question (ii)
What does the term ‘Little remains’ convey?
Answer:
Ulysses realizes that he has become old. He has not much time left. He doesn’t want to die resting in his kingdom. He states that his remaining lifetime is very limited.
Question (d)
“This is my son, mine own Telemachus,
To whom I leave the sceptre and the isle
Well-loved of me,”
Question (i)
Who does Ulysses entrust his kingdom to, in his absence?
Answer:
Ulysses entrusts his kingdom to his beloved son Telemachus in his absence.
Question (ii)
Bring out the significance of the ‘sceptre’.
Answer:
Sceptre is an ornamental staff carried by a King on ceremonial occasions as a symbol of sovereignity. It symbolizes the power of a king.
(e) ‘‘That ever with a frolic welcome took
The thunder and the sunshine, and opposed”
Question (i)
What do ‘thunder’ and ‘sunshine’ refer to?
Answer:
Thunder and sunshine refers to misfortunes and happy days. Ulysses and his comrades had undergone both kinds of experiences.
Question (ii)
What do we infer about the attitude of the sailors?
Answer:
The sailors shared the undying quest for exploration, adventure and for seeking newer knowledge in the untravelled world. They even welcomed dangers in fighting with Gods. They enjoyed the thrill of action and never worried about the outcome of battles or quests. They have equal temper of heroic hearts.
(f) ‘‘Death closes all: but something ere the end,
Some work of noble note, may yet be done,
Not unbecoming men that strove with Gods.”
Question (i)
The above lines convey the undying spirit of Ulysses. Explain.
Answer:
Ulysses is aware of ageing and substantial decrease in his physical strength. He knows that will close in on him sooner or later. But before that happens, he wants to sail beyond the sunset/horizon and if possible meet warriors like Achilles. He wants to achieve something worthy of those who challenged and fought with Gods. Thus these lines show the undying spirit of Ulysses.
Question (ii)
Pick out the words in alliteration in the above lines,
Answer:
ere, end, noble, note are the words that alliterate.
(g) “……… for my purpose holds
To sail beyond the sunset, and the baths Of all the western stars, until I die.”
Question (i)
What was Ulysses’ purpose in life?
Answer:
Ulysses proposes to sail beyond sunset and baths. His goal is not death but is in death. He seeks life in death. Ordinary mortals can’t reach ‘Happy isles’ or baths while they are alive.Ulysses wants to find direct evidence of spiritual reality after death. He wants to venture into the unknown.
Question (ii)
How long would his venture last?
Answer:
His venture would last until he confronts his death.
Question (h)
“One equal temper of heroic hearts,
Made weak by time and fate, but strong in will
To strive, to seek, to find, and not to yield. ”
Question (i)
Though made weak by time and fate, the hearts are heroic. Explain.
Answer:
Ulysses and his compatriots have visited many strange places in their previous voyages and enjoyed misfortunes and glorious triumphs with the same heroic temperament. They might have become old and may not have the same strength they had in youth. But they still share the thirst for travel and pursuit of knowledge in the unexplored world. Their bravery and spiritual strength are intact.
Question (ii)
Pick out the words in alliteration in the above lines.
Answer:
Strive, seek, heroic, hearts are the words that alliterate.
5. Explain with reference to the context the following lines.
Question (a)
“I cannot rest from travel: I will drink
Life to the lees:”
Answer:
Reference: These lines are from the poem ‘Ulysses” written by Alfred Tennyson.
Context and Explanation: Ulysses, after spending many years in the seas returns to Ithaca and starts ruling his country. But his heart is not in the administration of his kingdom. He wants to sail again. At this context he says these words. He wishes to enjoy life to the fullest and so he can’t afford to idle away his remaining life as a king.
Question (b)
“I am become a name;
For always roaming with a hungry heart”
Answer:
Reference: These lines are from the poem ‘Ulysses” written by Alfred Tennyson.
Context and Explanation: Ulysses says these words while discussing the reputation he has earned among the common multitude due to his daring adventures. He has roamed the world like a hungry lion. This line has a biblical alusion as well “Blessed are they which do hunger and thirst after righteousness” – Matthew V. 6.
Though Ulysses is aware of his fame, it doesn’t motivate him to stay or settle down in the kingdom of Ithaca. His inquisitive spirit is always looking for newer knowledge through ‘the arch’ to the untravelled world.
Question (c)
“How dull it is to pause, to make an end,
To rust unburnished, not to shine in use!”
Answer:
Reference: These lines are from the poem ‘Ulysses” written by Alfred Tennyson.
Context and Explanation: The poet says these words while discussing the mental agony of Ulysses who is unable to settle down with his ageing wife Penelope and son Telemachus. Ulysses finds doling out justice to a savage people as ‘boring’. He does not want to settle down and die in Ithaca. He compares himself to a sword which may rust if left unused. He wants to lead an active and adventurous life till his death.
Question (d)
“To follow knowledge like a sinking star,
Beyond the utmost bound of human thought”
Answer:
Reference: These lines are from the poem ‘Ulysses” written by Alfred Tennyson.
Context and Explanation: The poet says these words while describing the quest Ulysses has for adventure and fulfillment. Similar to a sinking star, Ulysses wants to pursue in his failing old age to pursue knowledge like the goal of Goethe’s Faust, his quest is defined by the pursuit of new and unique knowledge “beyond the utmost bound of human thought”.
Question (e)
“He works his work, I mine.’’’’
Answer:
Reference: These lines are from the poem ‘Ulysses” written by Alfred Tennyson.
Context and Explanation: The poet says these words while justifying the decision of Ulysses to pass on his kingdom to Telemachus. Ulysses explains the polar difference between himself and his son Telemachus. His son will be a ‘fair’ and ‘decent’ ruler. Unlike Ulysses, Telemachus is rooted in regular political life. He enjoys leading “savage” population and the responsibility of showing the subject better moral codes of conduct and upholding justice. Whereas Ulysses finds this “slow” and intolerable. So, he wishes his son to rule Ithaca and for himself he wishes to set sail to the unknown.
Question (f)
“….you and I are old;
Old age hath yet his honour and his toil;”
Answer:
Reference: These lines are from the poem ‘Ulysses” written by Alfred Tennyson.
Context and Explanation: Poet, in this part of the monologue describes the address made by Ulysses to his compatriots who were with him during “thunders and sunshine”. He admits the fact that they are growing old. But he does not want to retire like ordinary mortals. He accepts gracefully the honour befitting old age as a result of varied cultural experiences. Yet, he does not want old people to bow out of the field of action. He sincerely believes there is more work to be done, lands to be explored and newer knowledge to be acquired in old age before death.
Question (g)
“The long day wanes: the slow moon climbs: the deep
Moans round with many voices.”
Answer:
Reference: These lines are from the poem ‘Ulysses” written by Alfred Tennyson.
Context and Explanation: The poet poet continues to discuss old age and the tantalising call of the oceans to conquer. Ulysses hints at the probable end of the cycle of life in the words “The long clay wanes”. The symbol of darkness or night is mostly associated with death. The lure of ocean to resume his voyages beyond the point of sunset is too tempting to resist. The dark unfathomable sea beckons him and his compatriots with mysterious voices.
Question (h)
“It may be we shall touch the Happy Isles,
And see the great Achilles, whom we knew.”
Answer:
Reference: These lines are from the poem ‘Ulysses” written by Alfred Tennyson.
Context and Explanation: The poet says these words through Ulysses as to the probable outcome of the daring voyage at the fag end of his life. Ulysses is uncertain about the probable outcome of his last voyage. But he infers that he might reach ‘Happy Isles’ and see the ‘great Achilles’ who was dipped in the river of life.
Question (i)
“We are not now that strength which in old days
Moved earth and heaven;”
Answer:
Reference: These lines are from the poem ‘Ulysses” written by Alfred Tennyson.
Context and Explanation: The poet says these words through Ulysses when he wants to justify the reasons for resuming the daring voyage. He admits the decline in the compatriots’ physical strength with which they were able to move heaven and earth in their youth. He asks his compatriots to ignore the infinity of age and draw on their inner spiritual strength to resume their voyage beyond sunset.
Question (j)
“To strive, to seek, to find, and not to yield.”
Answer:
Reference: These lines are from the poem ‘Ulysses” written by Alfred Tennyson.
Context and Explanation: Tennyson says these words through Ulysses who makes his motto loud and clear in these words. The final line of the poem is Ulysses’ enduring challenge to readers as well. The challenge if the aged ones could push ahead with vigour and strength of will no matter how fragile their bodies are. To yield to age or weakness is to be less than fully human. It might be honourable to live a peaceful settled life in the old age. But one would naturally miss out most exciting moments of life if one does not venture out, at least a little towards the unknown.
6. Answer the following questions in a paragraph of about 100 words each.
Question (a)
What makes Ulysses seek newer adventures?
Answer:
In the context of the poem, Ulysses has grown old. He has experienced all daring adventures. He has won the hearts of people during the battle at Troy. Back home, as per the prophecy of Tiresias, he rules Ithaca for a brief time. But he is fed up with a conventional duties of a king. He laments his own uselessness as a ruler of idle people who lead life like savages, just eating and sleeping. They don’t understand the over vaulting ambition of their adventurous king Ulysses who had moved earth and heavens in the past. He wishes to embark upon his next voyage. It might be his last. He is quite sensitive to the moans of the seas tantalising him and his compatriots to set sail quickly. He wants “to drink life to the lees”. Ulysses doesn’t want to bask on the glory he has earned in the past.
His inquisitive spirit is restless. He has seen much’ and acquired knowledge of various cultures of the world. But he considers all such experiences like an “arch” leading him to the unexplored or “untravelled world”. He wants to sail towards the area ‘beyond sunset’. He must shine in use like a sword but not “rust unbumished”. Yet at home, in the kingdom of Ithaca, he feels bored and yearns to truly engage with what is left of life. He is impatient for “new” experiences lamenting everyday and every hour to seek “something more”. His quest for adventure and fulfillment, like the goal of Goethe’s Faust is defined by the pursuit of new knowledge “beyond the utmost bound of human thought”.
Question (b)
List the roles and responsibilities Ulysses assigns to his son Telemachus, while he is away.
Answer:
The entire poem is a monologue. Yet the second part of the poem is an address to the readers justifying his decision to transfer the rule to his son Telemachus. The cloak of a king seems to be unfit for the temperament of Uly sses. He finds ruling Ithaca a boring thing. He finds Telemachus rooted to the political life of Ithaca. His role is merely to lead a ‘savage race’ to accept standard norms of behaviour in the society. He believes Telemachus fits well with the role of the ruler of “uninspired and imprudent citizens” and may discharge his duties with honour and grace. When he is away, he wants his son Telemachus to dispense variable justice to the subjects of Ithaca and guide them in the path of virtues and morals.
Question (c)
What is Ulysses’ clarion call to his sailors? How does he inspire them?
Answer:
In the third part of the poem, Ulysses makes a clarion call to his hearty compatriots (i.e.) mariners. They have been with him both during ‘thick and thin’ or thunders or sunshine. Similar to Ulysses they possess “free hearts and free foreheads” (i.e.) their hearts and brains are unburdened by domestic cares and resposibilities. They had frolicsome time fighting along with Ulysses against great warriors and Gods in the past. Ulysses does not want to live in memory of glory. He believes they need not waste away their precious time in nostalgic memories just recounting their escapades to younger generation. They can really do ‘ something of noble note’ before the end. He is conscious of the impending death in old age. But he tells it is not “too late to seek a newer world”.
The many “voices of the ocean” call out to the mariners to resume voyage. Ulysses is not content with having earned a name for himself. He has seen many countries and acquired knowledge of various cultures. Those experiences are not to be taken as accomplishments. They are just an ‘arch’leading them to “untravelled world” and constantly sailing to the ever expanding horizon. He does not want his compatriots to miss even an hour which could prrovide them novel experiences in their voyage. He persuades his compatriots to gather at the port as the sails are already puffing up welcoming them all. Their life would be one of fulfillment only when they venture out into the unkown on the seas. He uses an emotional bait to his mariners. He highlights the probable outcome of their voyage. They might reach the “Happy Isles” (i.e.) great paradise and meet Achilles, their war hero. No matter how much strength they have, they still have some “strength of will” left to strive, to seek, to find, and not to yield.
Listening Activity
Listen to the poem and fill in the blanks with appropriate words and phrases. If required listen to the poem again.
Wander-thirst
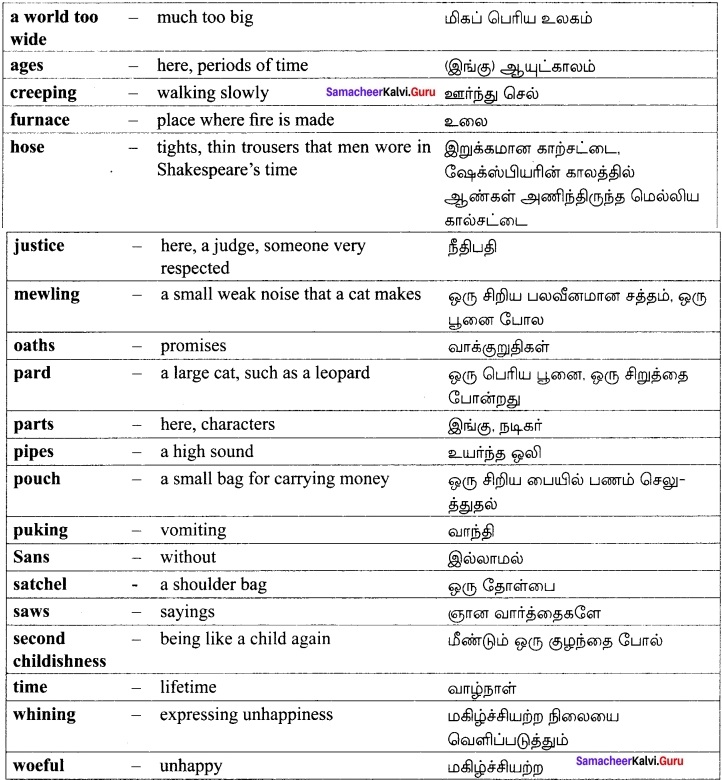
BEYOND the East the sunrise, beyond the West the sea, And East and West the wander-thirst that will not let me be; It works in me like madness, dear, to bid me say good-bye; For the seas call, and the stars call, and oh! the call of the sky!
I know not where the white road runs, nor what the blue hills are; But a man can have the sun for a friend, and for his guide a star; And there’s no end of voyaging when once the voice is heard, For the rivers call, and the roads call, and oh! the call of the bird!
Yonder the long horizon lies, and there by night and day The old ships draw to home again, the young ships sail away; And come I may, but go I must, and, if men ask you why, You may put the blame on the stars and the sun and the white road and the sky.
Choose the best option and complete the sentences:
Question 1
works like madness in the poet.
(a) Wander – Thirst
(b) Bidding Farewell
(c) Eastern Sunrise
(d) Western Seas
Answer:
(a) Wander – Thirst
Question 2.
A man could choose as his guide.
(a) the sun
(b) the hills
(c) a star
(d) a bird
Answer:
(c) a star
Question 3.
There is no end of once the voice is heard.
(a) walking
(b) roaming
(c) talking
(d) voyaging
Answer:
(d) voyaging
Question 4.
The old ships return, while the young ships
(a) drift
(b) move
(c) sail
(d) wander
Answer:
(c) sail
Question 5.
The blame is on the sun, stars, the road and the
(a) hills
(b) trees
(c) seas
(d) sky
Answer:
(d) sky
Ulysses About The Poet

Alfred Tennyson was the poet laureate of England and Ireland during Queen Victoria ‘s reign. Tennyson excelled in writing short lyrics such as “Break, Break, Break“, “ Tears Idle Tears“, “The Charge of the Light Brigade” and.‘Crossing the Bar’. Tennyson’s use of the musical qualities of words to emphasise his rhythms and meanings is sensitive.
Ulysses Summary in English
Introduction
‘Ulysses’ is a dramatic monologue in which Ulysses, the king of Ithaca expresses his undying thirst of adventure overseas. Tennyson has written this poem in memory of Arthur Henry Hallam who died young. Death is not the end for both Arthur and Ulysses.
Thirst for adventure
Ulysses addresses mariners in the third part. Ulysses does not see any worth in staying in the comfort of family life with his wife. As a king, he listens to the complaints of people and gives rewards and punishments for the citizens of his country. He discloses his inner nature, “cannot rest from travel!” He has become a living icon of travel overseas. He wants “to drink life to the lees”. He confesses that he has both enjoyed and suffered a lot during his travels. He has gained profound wisdom during his travels and battles. Enriched by his new gained cultural knowledge, he longs to resume his voyages. He believes that to rest is rust. Every hour has the •potential to bring new knowledge. So, it is meaningless to stay.
Passing on the inheritance
In the second part addresses the reader explaining why Ulysses doesn’t want to continue to rule. Ulysses wishes to hand over his kingdom to his son Telemachus. He believes he would rule the kingdom and render appropriate justice to his subjects. Telemachus, unlike Ulysses, is rooted to the soil. He is kind to the subjects and addresses their needs. Besides, Ulysses hopes that Penelope, his aging wife would be happy to spend her last years with her son and grandchildren. So, Ulysses can resume his voyage along with his old friends.
Call to set sail
Ulysses called his old companions to the port where the ship awaits them all. His old companions have fought many battles alongside him and share the undying quest for adventure. Ulysses persuades his friends to join him on his voyage to the edge of the world and beyond to find a new world. He knows the limitations of his companions. They may have grown old and weak due to age. Their physical powers may not be the same as when they had once moved heaven and earth. But he is confident that their spirit is young and undaunted. His clarion call would inspire invariably all those who seek knowledge and strive to lead meaningful lives.
Conclusion
People who are endowed with an unquenchable thirst for ever-expanding knowledge and incurable love for long distances Ulysses continues to be a source of inspiration.
Ulysses Summary in Tamil
முடிவுரை:
தன் அறிவு வேட்கையை அதிகப்படுத்துவதில் | ஆர்வம் கொண்டவர்களுக்கும் மற்றும் மாறாத தொலைதூரப் பயணத்தில் காதல் கொண்டவர்களுக்கும் | யுலிசஸ் எப்போதும் தூண்டுதலாக இருக்கிறார்.
சாகசத்தின் மேல் ஆவல்
யுலிசஸ் மூன்றாம் பாகத்தில் கப்பலோட்டிகளிடத்தில் சொற்பொழிவாற்றுகிறார். தன் குடும்பத்தாருடன் சொகுசு வாழ்க்கை வாழ்வதில் எந்தப் பயனும் இல்லை என எண்ணுகிறார். ஒரு அரசனாக குடிமக்களின் கூற்றுகளைக் கேட்டு அதற்கு ஏற்ற பரிசையும் மற்றும் தண்டனையும் வழங்குகிறார். கடற் பயணம் செய்யாமல் இருக்க முடியாது என்ற தன்னுடைய உள்ளுணர்வை வெளிப்படுத்துகிறார். கடல் கடந்த பயணங்களை மேற்கொள்ளும் உயிரோவியமாகத் திகழ்ந்தார். வாழ்க்கை முழுவதும் பயணிக்க விரும்புகிறார். அவர் பயணத்தில் மேற்கொண்ட இன்ப துன்பங்களை ஒப்புக் கொள்கிறார். அவர் பயணத்தின் போது ஏற்பட்ட அனுபவங்களாலும் மற்றும் எதிர்கொண்ட போர்களினாலும் ஆழ்ந்த ஞானத்தை அடைந்திருந்தார். புதிதாக பெறப்பெற்ற பண்புகளின் புலமையால், அவர் பயணங்களை மேற்கொள்வதில் ஆர்வத்தைக் காட்டினார். சோம்பலாக கிடந்தால் உடல் துருப்பிடித்து விடும் என எண்ணினார். ஒவ்வொரு கணமும் புதுப் பொலிவைக் கொண்டு வரும் ஆற்றல் மிக்கது. ஆதலால், ஓரிடத்தில் ஒண்டிக் கிடப்பதில் பயனில்லை என எண்ணினார்.
பரம்பரை சொத்துக்களை கைமாற்றம் செய்தல்:
இரண்டாம் பாக சொற்பொழிவில் அவருக்கு அரசாள்வதில் ஏன் விருப்பம் இல்லை என்பதைக் குறித்துக் கூறுகிறார். யுலிசஸ் தன் இராஜ்ஜியத்தை தன் புதல்வன் டெலிமேக்கஸ் (Tele Macus) இடம் கைமாற்றுகிறார். அவர் தன் புதல்வன் நல்ல முறையில் அரசாண்டு தன் குடிமக்களுக்கு தக்க நீதி வழங்குவான் என எண்ணினார். டெலிமாக்கஸ், யுலிசஸ் போல் அல்லாமல் ஒரே இடத்தில் ஊன்றி நிற்பவராய் இருந்தார். அவர்தம் குடிமக்களுக்கு அன்பார்ந்தவராகவும் அவர்கள் குறைகளைக் கேட்டறிபவராகவும் இருந்தார். அது தவிர யுலிசஸ் தன் வயது முதிர்ந்த மனைவி பெனைலோப்பிம், மகனுடனும் பேரப் பிள்ளைகளுடனும் மகிழ்ச்சியோடு இருப்பார் என நம்பினார். ஏனெனில், அது அவர் தன் தோழர்களுடன் கடல் பயணம் மேற்கொள்ளத் தக்கவாறு அமையும்.
கடல் பயணம் மேற்கொள்ளல்:
யுலிசஸ் தன் நண்பர்களைத் தமக்காக காத்துக் கொண்டிருக்கும் துறைமுகத்திற்கு வருமாறு அழைப்பு விடுகிறார். அவர் தோழர்களும் கடல் பயணத்தின் போது பல கடற்போர்களில் ஈடுபட்டு தனக்கு உள்ள கடல் பயணத்தின் மேல் உள்ள ஆவலைப் பகிர்ந்து கொண்டனர். உலகின் விளிம்பை அடைந்து அதற்கு அப்பாலும் செல்வதற்கு தன்னுடன் கடல் பயணம் மேற்கொள்ள நண்பர்களைத் தூண்டுகிறார். அவர் தம் நண்பர்களின் குறைகளை அறிந்திருந்தார். அவர்கள் வயது முதிர்ச்சியால் பலவீனமாகக் காணப்பட்டனர். அவர்கள், முன்னர் வானத்தையும், பூமியையும் அளந்தது போல் அவர்கள் உடல் வலிமை தற்போது இல்லை. ஆனால், அவர்கள் ஊக்கம் குன்றாமல், சளைக்கா வண்ணம் தொடரவேண்டும் என்பதில் உறுதியாக இருந்தார். அவரின் வீர முழக்கம் புதிய அனுபவத்தை நாடி மற்றும் அர்த்தமுள்ள வாழ்க்கையை வாழ முயற்சிக்கும் அனைவருக்கும் தூண்டுதலாக இருக்கும்.
முன்னுரை:
யூலிஸ் என்பது வியத்தகு மோனோலாலஜி ஆகும். இதில் இதாகா மன்னனான யுலிசஸ் வெளிநாடுகளில் சாகசத் தன்மையற்ற தாகத்தை வெளிப்படுத்துகிறார். டென்னிசன் இளம் கவிஞரான ஆர்தர் ஹென்றி ஹாலமின் நினைவாக இந்தக் கவிதை எழுதினார். ஆர்தர் மற்றும் யுலிசஸ் இருவருக்கும் முடிவே இல்லை.
Ulysses Glossary
Textual:
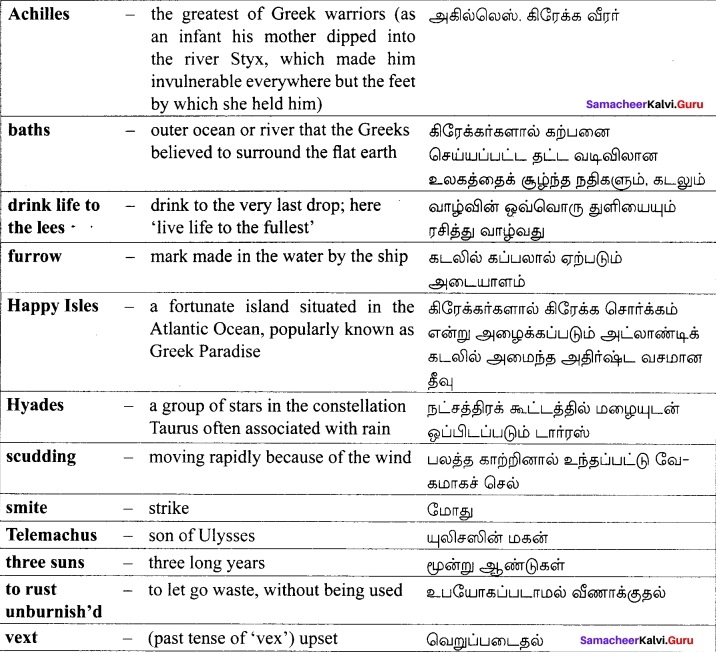
Additional: