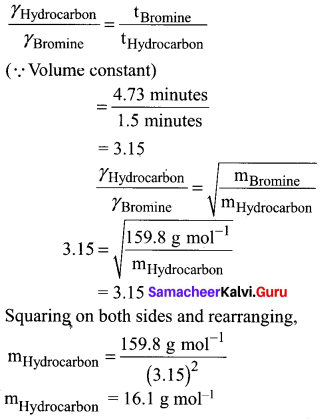
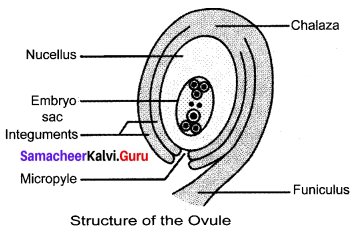
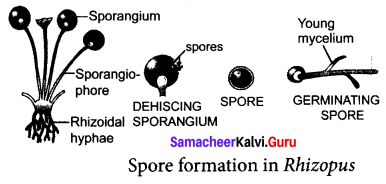
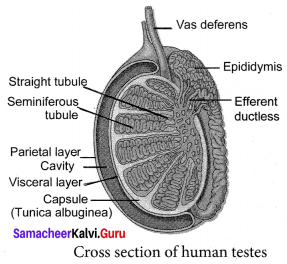
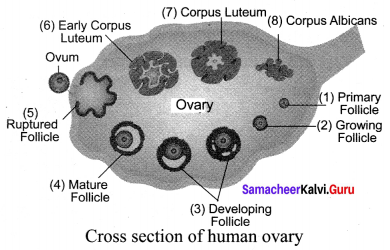
You can Download Samacheer Kalvi 9th Social Science Book Solutions Guide Pdf, Tamilnadu State Board help you to revise the complete Syllabus and score more marks in your examinations.
Tamilnadu Samacheer Kalvi 9th Social Science Civics Solutions Chapter 2 Election, Political Parties and Pressure Groups
Election, Political Parties and Pressure Groups Textual Exercise
I. Choose the correct answer.
Election Political Parties And Pressure Groups Question 1.
India has adapted the electoral system followed in the
(a) USA
(b) United Kingdom
(c) Canada
(d) Russia
Answer:
(b) United Kingdom
Election Political Parties And Pressure Groups Questions And Answers Question 2.
The Election Commission of India is a/an
(a) Independent body
(b) Statutory body
(c) Private body
(d) Public corporation
Answer:
(a) Independent body
Class 9 Civics Chapter 2 Question 3.
Which Article of the Constitution provides for an Election Commission?
(a) Article 280
(b) Article 315
(c) Article 324
(d) Article 325
Answer:
(c) Article 324
Political Parties And Pressure Groups Question 4.
Which part of the constitution of India says about the election commission?
(a) Part III
(b) Part XV
(c) Part XX
(d) Part XXII
Answer:
(b) Part XV
Chapter 2 Civics Class 9 Question 5.
Who accords recognition to various political parties as national or regional parties?
(a) The President
(b) The Election Commission
(c) The Parliament
(d) The President in consultation with the Election Commission
Answer:
(b) The Election Commission
Question 6.
Assertion (A): Indian Constitution provides for an independent Election Commission
Reason (R): To ensure free and fair elections in the country.
(a) Both (A) and (R) are true and (R) explains (A)
(b) Both (A) and (R) are true and (R) does not explain (A)
(c) (A) is correct and (R) is false
(d) (A) is false and (R) is true
Answer:
(a) Both (A) and (R) are true and (R) explains (A)
Question 7.
NOT A was introduced in the year ………..
(a) 2012
(b) 2013
(c) 2014
(d) 2015
Answer:
(c) 2014
Question 8.
The term pressure groups originated in …….
(a) USA
(b) UK
(c) USSR
(d) India
Answer:
(a) USA
Question 9.
Assertion (A): A large number of pressure groups exist in India.
Reason (R): Pressure Groups are not developed in India to the same extent as in the USA.
(a) Both (A) and (R) are true and (R) explains (A)
(b) Both (A) and (R) are true and (R) does not explain (A)
(c) (A) is correct and (R) is false
(d) (A) is false and (R) is true
Answer:
(a) Both (A) and (R) are true and (R) explains (A)
II. Fill in the blanks.
1. The Election Commission of India is a body of ……… members.
2. National Voters day has been celebrated on ………
3. In India ……… party system is followed.
4. In 2017, there were ……. recognised national parties.
5. Narmada Bachao Andolan is a …………
Answers:
1. 3
2. 25th January
3. Multi
4. Seven
5. Pressure group
III. Match the following.

Answers:
1. (d)
2. (c)
3. (b)
4. (a)
IV. Give short answers.
Question 1.
Explain the electoral system in India.
Answer:
- The electoral system in India has been adapted from the system followed in the United Kingdom.
- India is a socialist, secular, democratic, republic and the largest democracy in the world.
- The modem Indian nation state came into existence on 15th August 1947.
Question 2.
Give the meaning of a political party.
Answer:
A political party is an organisation formed by a group of people with a certain ideology and agenda to contest elections and hold power in the government.
A political party has three components: a leader, active members and the followers.
Question 3.
Distinguish between two-party system and the multi-party system.
Answer:
| Two party system | Multi-party system |
| Two party system in which only two major parties exist, for example, USA, UK. | Multi-party system in which there are more than two political parties, for example India, Srilanka, France and Italy. |
Question 4.
What is a pressure group?
Answer:
- A pressure group is a group of people who are organised actively for promoting and defending their common interest. It is so called as it attempts to bring a change in the public policy by exerting pressure on the government.
- The pressure groups are also called ‘interest groups’ or vested groups.
- They are different from the political parties in that they neither contest elections nor try to capture political power.
V. Answer in detail.
Question 1.
Discuss merits and demerits of direct elections?
Answer:
Merits:
- As the voters elect their representatives directly, direct elections are considered to be a more democratic method of election.
- It educates people regarding the government activities and helps in choosing the appropriate candidates. Also, it encourages people to play an active role in politics.
- It empowers people and makes the rulers accountable for their actions.
Demerits:
- Direct elections are very expensive.
- Illiterate voters sometimes get misguided by false propaganda and sometimes campaigning based on caste, religious and various other sectarian consideration spose serious challenges.
- Since conducting direct elections is a massive exercise, ensuring free and fair elections at every polling station is a major challenge to the Election Commission.
- There are instances of some political candidates influencing the voters through payments in the form of cash, goods or services.
- Election campaigns sometimes results in violence, tension, law and order problems and
affects the day-to-day life of people.
Question 2.
What are the functions of political parties?
Answer:
- Parties contest elections. In most democracies, elections are fought mainly among the candidates put up by political parties.
- Parties put forward their policies and programmes before the electorate to consider and choose.
- Parties play a decisive role in making laws for a country. Formally, laws are debated and passed in the legislature.
- Parties form and run the governments.
- Those parties that lose in the elections play the role of the Opposition to the party or a group of coalition parties in power, by voicing different views and criticising the government for its failures or wrong policies.
- Parties shape public opinion. They raise and highlight issues of importance.
- Parties function as the useful link between people and the government machinery.
Question 3.
What are the functions of Pressure groups in India?
Answer:
Pressure groups are the interest groups that work to secure certain interest by influencing the public policy. They are non-aligned with any political party and work as an indirect yet powerful group to influence the policy decisions. Pressure groups carry out a range of functions including representation, political participation, education, policy formulation and policy implementation.
Political Participation: Pressure groups can be called the informal face of politics. They exert influence precisely by mobilising popular support through activities such as petitions, marches, demonstrations and other forms of political protest. Such forms of political participation have been particularly attractive to young people.
Education: Many pressure groups devote significant resources by carrying out research, maintaining websites, commenting on government policy and using high-profile academics, scientists and even celebrities to get their views across, with an emphasis to cultivate expert authority.
Policy Formulation: Though the pressure groups themselves are not policy-makers, yet it does not prevent many of them from participating in the policy-making process. Many pressure groups are vital sources of information and render advice to the government and therefore they are regularly consulted in the process of policy formulation.
VI. Project and Activity
Question 1.
Compare the policies, programmes and achievements of a national party and a state party.
Answer:
- Refer the National policies, programmes and achievements from the Internet and library books.
- The students are instructed to compare the policies programmes and achievements.
- This is a group Activity.
VII. HOTS
Question 1.
“Elections are considered essential for any representative democracy”. Why?
Answer:
“A democracy requires a mechanism by which people can choose their representatives at regular intervals and change them if they wish to do so. Therefore, elections are considered essential for any representative democracy. In an election the voters make many choices.
- This helps the public in choosing the development course which they want and want to adopt.
- This directly provide the public the opportunity to select their representatives and these representatives decision can be legitimacy.
- This also ensures the transparency as well as the accountability as the public representatives are chosen directly.
Question 2.
What is the principle of universal adult franchise? What is its importance?
Answer:
Principle: Universal Adult Franchise means that the right to vote should be given to all adult citizens without the discrimination of caste, class, colour, religion (or) gender. It is based on equality, which is a basic principle of democracy.
Importance: Under this system a government is elected that is accountable to the people it governs. Because every vote counts, issues in a society receive their appropriate weight in terms of importance and urgency.
Question 3.
Discuss merits and demerits of democracy.
Answer:
| Merits | Demerits | ||
| 1. | Safeguards the interests of the people. | 1. | More emphasis on quantity than on quality. |
| 2. | Based on the principle of equality. | 2. | Rule of the incompetent. |
| 3. | Stability and responsibility in administration. | 3. | Based on unnatural equality. • |
| 4. | Political education to the people. | 4. | Voters do not take interest in election. |
| 5. | Little chance of revolution. | 5. | Lowers the moral standard. |
| 6. | Stable government. | 6. | Democracy is a government of the rich. |
| 7. | Helps in making people good citizens. | 7. | Misuse of public funds and time. |
| 8. | Based on public opinion. | 8. | No stable government. |
| 9. | Dictatorship of majority. | ||
| 10. | Bad influence of political parties. |
Question 4.
Discuss the multi-party system.
Answer:
- A multi-party system is a system where multiple political parties that have ideas participate in the national elections.
- A lot of countries that use this system have a coalition government, meaning many parties are in control, and they all work together to make laws.
- Countries with a multi-party political system tend to have greater voter participation.
- No democracy can survive without multi-party system.
VIII. Life Skill
Question 1.
Conduct a mock poll in your classroom.
Answer:
- Help the students to understand the process of electing officials and the power of vote by holding a mock election.
- These are great activities to enjoy during the presidential election.
- Students explain the steps taken from party formation to National election.
- The students will act out the campaigning and voting process by stimulating a real election in their own class room.
Steps set for a mock-poll in the class room:
- Setting up the political parties.
- Preparing a manifesto.
- Running a campaign.
- Holding the class room election.
Election, Political Parties and Pressure Groups Additional Questions
I. Choose the correct answer.
Question 1.
India is the democracy in the world.
(a) largest
(b) smallest
(c) strongest
(d) None of the above
Answer:
(a) largest
Question 2.
Kudavolai was the system of voting followed during the ……. period in Tamil Nadu.
(a) Chera
(b) Chola
(c) Pandya
(d) Pallava
Answer:
(b) Chola
Question 3.
Which country has single party system?
(a) USA
(b) UK
(c) Cuba
(d) France
Answer:
(c) Cuba
Question 4.
Assertion (A): Parties shape public opinion.
Reason (R): They raise and highlight issues of importance.
(a) Both (A) and (R) are true and (R) explains (A)
(b) Both (A) and (R) are true and (R) does not explain (A).
(c) (A) is correct and (R) is false
(d) (A) is false and (R) is true
Answer:
(a) Both (A) and (R) are true and (R) explains (A)
Question 5.
India is the ………… th country in the world to introduce NOTA.
(a) 10
(b) 12
(c) 14
(d) 16
Answer:
(c) 14
Question 6.
The ……. is elected by members of the Lok Sabha.
(a) Prime Minister
(b) President
(c) Governor
(d) Cabinet Minister
Answer:
(a) Prime Minister
II. Fill in the blanks.
1. ……. in elections are the best way to make your ‘voice’ heard.
2. Indirect elections are less ……..
3. ……… parties are an essential part of Democracy.
4. …… treats all the parties equally.
5. The pressure groups are also called ……. groups.
6. A political party has three components: a ………. and the ……….
Answers:
1. Voting
2. expensive
3. Political
4. Election Commission
5. Interest
6. a leader, acting members, followers
III. Match the following.

Answers:
1. (d)
2. (a)
3. (b)
4. (c)
IV. Give short answers.
Question 1.
What do you know about Voters Verified Paper Audit Trail?
Answer:
- Voters Verified Paper Audit Trail (WPAT) is the way forward to enhance credibility and transparency of the election process.
- This system was first introduced in 2014 General election.
Question 2.
Mention the merits and demerits of Indirect elections.
Answer:
Merits:
- Indirect elections are less expensive.
- It is more suited to elections in large countries.
Demerits:
- If the number of voters is very small, there exists the possibility of corruption, bribery, horse trading and other unfair activities.
- It is less democratic because people do not have a direct opportunity to elect, but they instead do it through their representatives. So, this may not reflect the true will of the people.
Question 3.
Write a short note on “State Parties”.
Answer:
Other than the seven national parties, most of the major parties of the country are classified by the Election Commission as ‘state parties’. These are commonly referred to as regional parties. A party is recognised as a state party by the Election Commission of India based on certain percentage of votes secured or a certain number of seats won in the Assembly or Lok Sabha elections.
Question 4.
Classify pressure groups in India.
Answer:
The pressure groups in India can be broadly classified into the following categories:
- Business groups
- Trade unions
- Agrarian groups
- Professional associations
- Student organisations
- Religious organisations
- Tribal organisations
- Linguistic groups
- Ideology-based groups
- Environmental protection groups.
V. Answer in detail.
Question 1.
Give an account of Mobilisation and Democratic Participation.
Answer:
Mobilising people towards socially productive activities that lead to the overall betterment of people’s lives is essential. Sometimes earthquakes, tsunamis, floods and other such natural disasters on a massive scale occur and people’s immediate mobilisation for evacuation and emergency relief becomes most essential.
Democratic Participation: Democracy can-succeed only when smaller local groups and, in fact, every citizen can take action that supports the tax and revenue collection systems, observance of national norms in environmental protection, cleanliness, health and hygiene, sanitary drives and immunisation programmes like pulse polio.
However, we must keep in mind that there is no better form of government than Democratic government. To create a better society and nation, the people of India along with the union and state governments should come together to fight against the miseries of human life.