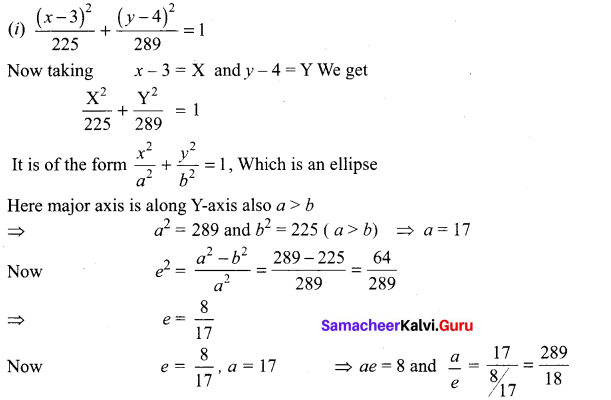
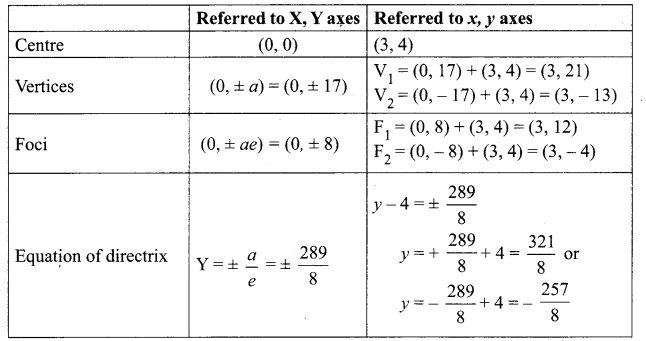
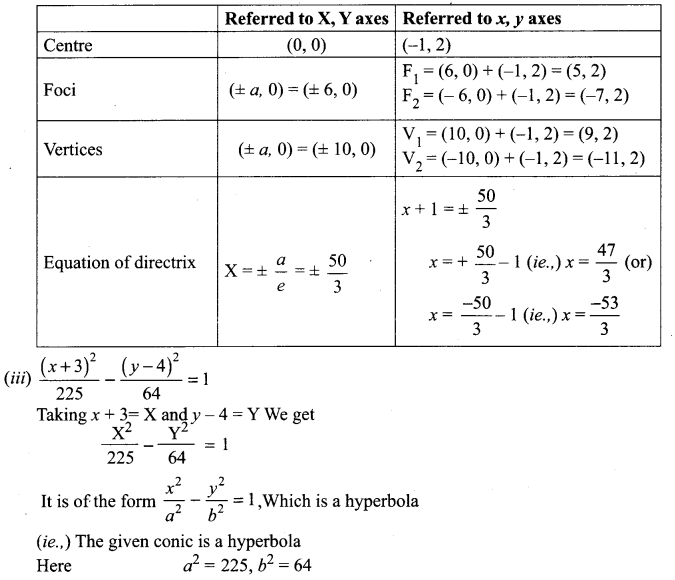
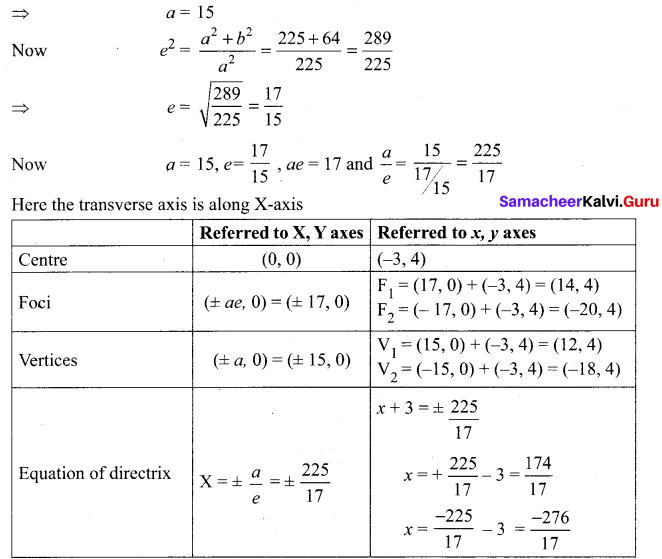
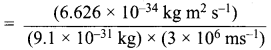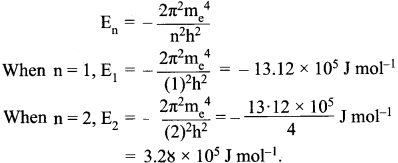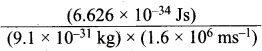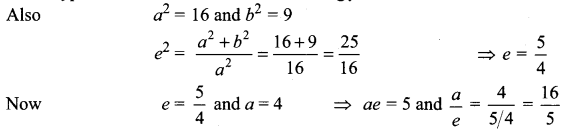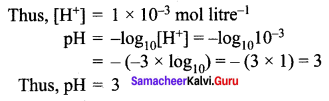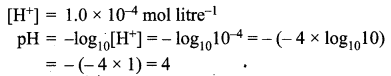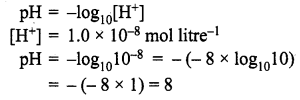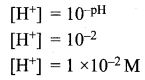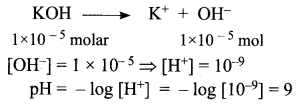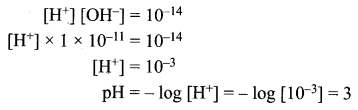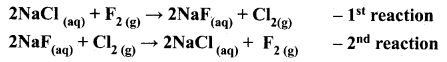Tamilnadu State Board New Syllabus Samacheer Kalvi 8th Tamil Book Solutions Guide Pdf Chapter 3.1 நோயும் மருந்தும் Text Book Back Questions and Answers, Summary, Notes.
Tamilnadu Samacheer Kalvi 8th Tamil Solutions Chapter 3.1 நோயும் மருந்தும்
கற்பவை கற்றபின்
Question 1.
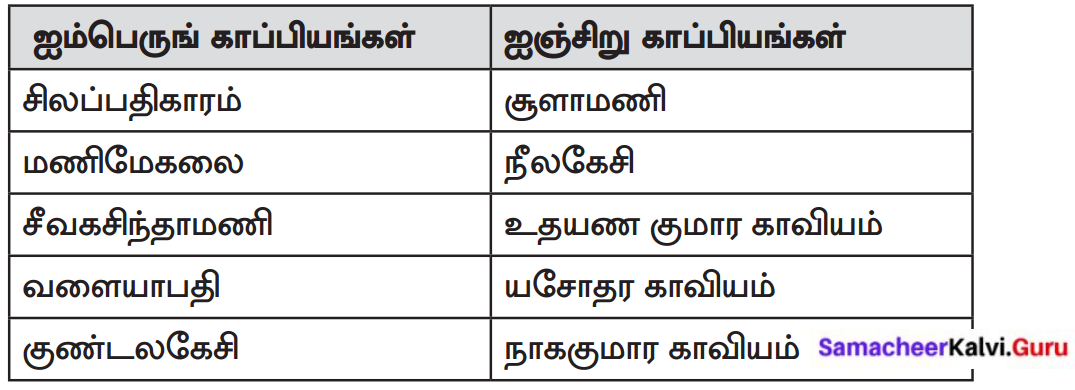
ஐம்பெருங்காப்பியங்கள், ஐஞ்சிறுகாப்பியங்கள் ஆகியவற்றின் பெயர்களைத் தொகுத்து எழுதுக.
Answer:
மாடநூல் வினாக்கள்
சரியான விடையைத் தேர்ந்தெடுத்து எழுதுக.
Question 1.
உடல்நலம் என்பது ……………………… இல்லாமல் வாழ்தல் ஆகும்.
அ) அணி
ஆ) பணி
இ) பிணி
ஈ) மணி
Answer:
இ) பிணி
Question 2.
நீலகேசி கூறும் நோயின் வகைகள் ……………………
அ) இரண்டு
ஆ) மூன்று
இ) நான்கு
ஈ) ஐந்து
Answer:
ஆ) மூன்று
Question 3.
‘இவையுண்டார்’ என்னும் சொல்லைப் பிரித்து எழுதக் கிடைப்பது_ ………………………
அ) இ + யுண்டார்
ஆ) இவ் + உண்டார்
இ) இவை + உண்டார்
ஈ) இவை + யுண்டார்
Answer:
இ) இவை + உண்டார்
Question 4.
தாம் + இனி என்பதனைச் சேர்த்தெழுதக் கிடைக்கும் சொல் ……………………
அ) தாம் இனி
ஆ) தாம்மினி
இ) தாமினி
ஈ) தாமனி
Answer:
இ) தாமினி
குறுவினா
Question 1.
நோயின் மூன்று வகைகள் யாவை?
Answer:
- மருந்தினால் நீங்கும் நோய்.
- எதனாலும் தீராத தன்மையுடைய நோய் மற்றொரு வகை.
- வெளியில் ஆறி உள்ளுக்குள் இருந்து துன்பம் தரும் நோய்.
Question 2.
நீலகேசியில் பிறவித் துன்பத்தைத் தீர்க்கும் மருந்துகளாகக் கூறப்படுவன யாவை?
Answer:
நல்லறிவு, நற்காட்சி, நல்லொழுக்கம் என்பவையே பிறவித் துன்பத்தைத் தீர்க்கும் மருந்துகளாக நீலகேசி கூறுகின்றது.
சிறு வினா
Question 1.
நோயின் வகைகள் அவற்றைத் தீர்க்கும் வழிகள் பற்றி நீலகேசி கூறுவன யாவை?
Answer:
- ஒளிபொருந்திய அணிகலன்களை அணிந்த பெண்ணே! நோயின் தன்மை பற்றி யார் வினவினாலும் அது மூன்று வகைப்படும் என அறிவாயாக.
- மருந்தினால் நீங்கும் நோய்.
- எதனாலும் தீராத தன்மையுடைய நோய் மற்றொரு வகை.
- வெளியில் ஆறி உள்ளுக்குள் இருந்து துன்பம் தரும் நோய்.
- அகற்றுவதற்கு அரியவை பிறவித் துன்பங்கள் ஆகும்.
- இவற்றைத் தீர்க்கும் மருந்துகள் மூன்று. நல்லறிவு, நற்காட்சி, நல்லொழுக்கம் என்பவையே அம்மருந்துகள்.
- இவற்றை ஏற்றோர் பிறவித்துன்பத்திலிருந்து நீங்கி உயரிய இன்பத்தை அடைவர்.
சிந்தனை வினா
Question 1.
துன்பமின்றி வாழ நாம் கைக்கொள்ள வேண்டிய நற்பண்புகள் யாவை?
Answer:
தருமம் செய்தல், கோபத்தைத் தணித்தல், முயற்சி செய்தல், கல்வி கற்றல், உலக நடையை அறிந்து நடத்தல், நல்ல நூல்களைப் படித்தல், பொறாமை படாமல் இருத்தல், பொய்சாட்சி சொல்லாமல் இருத்தல், இனிமையாகப் பேசுதல், பேராசையைத் தவிர்த்தல், நட்புடன் பழகுதல், பெரியோர்களை மதித்தல், ஒழுக்கம் தவறாமல் இருத்தல், நன்றியை மறவாமல் இருத்தல், காலத்தைக் கடைபிடித்தல், களவு செய்யாதிருத்தல், இழிவானதைச் செய்யாதிருத்தல், இரக்கம் கொள்ளுதல், பொய் சொல்லாதிருத்தல், ஆணவம் கொள்ளாதிருத்தல், சுறுசுறுப்புடன் இருத்தல், உடற்பயிற்சி செய்தல், அதிகாலையில் எழுந்திருத்தல் போன்றவை கைக்கொள்ள வேண்டிய நற்பண்புகள் ஆகும்.
கூடுதல் வினாக்கள்
சரியான விடையைத் தேர்ந்தெடுத்து எழுதுக.
Question 1.
மக்களின் உடலுக்கும் உள்ளத்திற்கும் துன்பம் தருவன …………………….
அ) நாய்கள்
ஆ) நோய்கள்
இ) பேய்கள்
ஈ) மனிதர்கள்
Answer:
ஆ) நோய்கள்
Question 2.
உள்ளத்தில் தோன்றும் தீய எண்ணங்களால் ஏற்படும் துன்பங்களையும் ……………………… என்றே நம் முன்னோர்கள் குறிப்பிட்டனர்.
அ) கவலை
ஆ) துன்பம்
இ) நோய்கள்
ஈ) பொறுமை
Answer:
இ) நோய்கள்
Question 3.
நோய்களை நீக்கும் மருந்துகளாக விளங்கும் அறக்கருத்துகளை விளக்குபவை …………………..
அ) இலக்கியங்கள்
ஆ) இலக்கணங்கள்
இ) படைப்புகள்
ஈ) முன்னோர்கள்
Answer:
அ) இலக்கியங்கள்
Question 4.
நோயைத் தீர்க்கும் மருந்துகள் ………………….
அ) இரண்டு
ஆ) மூன்று
இ) நான்கு
ஈ) ஐந்து
Answer:
ஆ) மூன்று
Question 5.
………………… ஐஞ்சிறு காப்பியங்களுள் ஒன்று.
அ) சிலப்பதிகாரம்
ஆ) நீலகேசி
இ) குண்டலகேசி
ஈ) வளையாபதி
Answer:
ஆ) நீலகேசி
Question 6.
நீலகேசி ………………… சமயக் கருத்துகளைக் கூறுகிறது.
அ) சமணம்
ஆ) புத்தம்
இ) கிறித்தவம்
ஈ) இந்து
Answer:
அ) சமணம்
Question 7.
நீலகேசி, கடவுள் வாழ்த்து நீங்கலாக ……………….. சருக்கங்களைக் கொண்டது.
அ) எட்டு
ஆ) ஒன்பது
இ) ஏழு
ஈ) பத்து
Answer:
ஈ) பத்து
Question 8.
‘போலாதும்’ என்னும் சொல்லைப் பிரித்து எழுதக் கிடைப்பது ………………………..
அ) போ + தும்
ஆ) போல் + ஆதும்
இ) போல் + அனதும்
ஈ) போலா + தும்
Answer:
ஆ) போல்+ஆதும்
Question 9.
‘உய்ப்பனவும்’ என்னும் சொல்லைப் பிரித்து எழுதக் கிடைப்பது …………………
அ) உய் + பனவும்
ஆ) உய்ப் + பனவும்
இ) உய்ப்ப ன + உம்
ஈ) உய்ப்ப ன + அம்
Answer:
இ) உய்ப்பன+உம்
Question 10.
‘கூற்றவா’ என்னும் சொல்லைப் பிரித்து எழுதக் கிடைப்பது …………………….
அ) கூ + அவா
ஆ) கூற்று + அவா
இ) கூற் + அவா
ஈ) கூற்று + ஆவா
Answer:
ஆ) கூற்று+அவா
Question 11.
‘ஐம்பெருங்காப்பியம்’ என்னும் சொல்லைப் பிரித்து எழுதக் கிடைப்பது …………………..
அ) ஐந்து + காப்பியம்
ஆ) ஐந்து + பெரு + காப்பியம்
இ) ஐம்பெருங் + காப்பியம்
ஈ) ஐந்து + பெருமை + காப்பியம்
Answer:
ஈ) ஐந்து + பெருமை + காப்பியம்
Question 12.
‘அரும்பிணி’ என்னும் சொல்லைப் பிரித்து எழுதக் கிடைப்பது ………………………….
அ) அரும் + பிணி
ஆ) அரு + பிணி
இ) அருமை + பிணி
ஈ) அரும் + பணி
Answer:
இ) அருமை + பிணி
Question 13.
‘அ + பிணி’ என்பதனைச் சேர்த்தெழுதக் கிடைக்கும் சொல் ……………………….
அ) அபிணி
ஆ) அப்பிணி
இ) அப்பிணி
ஈ) அதுபிணி
Answer:
இ) அப்பிணி .
Question 14.
‘தெளிவு + ஓடு’ என்பதனைச் சேர்த்தெழுதக் கிடைக்கும் சொல் …………………..
அ) தெளிவுவூடு
ஆ) தெளிவோடு
இ) தெளிவுஓடு
ஈ) தெளிவாடு
Answer:
ஆ) தெளிவோடு
Question 15.
‘பிணி + உள்’ என்பதனைச் சேர்த்தெழுதக் கிடைக்கும் சொல் ……………….
அ) பிணியுள்
ஆ) பிணினள்
இ) பிணியாள்
ஈ) பிணிபுள்
Answer:
அ) பிணியுள்
Question 16.
‘இன்பம் + உற்றே’ என்பதனைச் சேர்த்தெழுதக் கிடைக்கும் சொல் ………………….
அ) இன்பமுற்று
ஆ) இன்பமற்றே
இ) இன்பம் உற்றோ
ஈ) இன்பமுற்றே
Answer:
ஈ) இன்பமுற்றே
குறுவினா
Question 1.
நோய்கள் எவற்றிற்கெல்லாம் துன்பம் தருவன?
Answer:
மக்களின் உடலுக்கும் உள்ளத்திற்கும் துன்பம் தருவன நோய்கள்.
Question 2.
நம் முன்னோர்கள் எவற்றையும் நோய்கள் என்று கூறினர்?
Answer:
உள்ளத்தில் தோன்றும் தீய எண்ணங்களால் ஏற்படும் துன்பங்களையும் நோய்கள் என்றே நம் முன்னோர் கூறினர்.
Question 3.
இலக்கியங்கள் விளக்குவன யாவை?
Answer:
நோய்களை நீக்கும் மருந்துகளாக விளங்கும் அறக்கருத்துகளை இலக்கியங்கள் விளக்குகின்றன.
Question 4.
ஐம்பெருங்காப்பியங்கள் யாவை?
Answer:
சிலப்பதிகாரம், மணிமேகலை, சீவகசிந்தாமணி, வளையாபதி, குண்டலகேசி.
Question 5.
ஐஞ்சிறுகாப்பியங்கள் யாவை?
Answer:
சூளாமணி, நீலகேசி, உதயண குமார காவியம், யசோதர காவியம், நாககுமார காவியம்.
சிறுவினா
Question 1.
நீலகேசி குறித்து எழுதுக.
Answer:
- நீலகேசி என்றால் கருத்த கூந்தலை உடையவள் என்று பொருள்.
- ஆசிரியர் பெயர் அறியப்படவில்லை .
- கடவுள் வாழ்த்து நீங்கலாகப் பத்துச் சருக்கங்களைக் கொண்டது.
- 894 பாடல்களைக் கொண்டது.
- நீலகேசி தெருட்டு என்ற வேறு பெயரும் உண்டு.
சொல்லும் பொருளும்
தீர்வன – நீங்குபவை
உவசமம் – அடங்கி இருத்தல்
நிழல் இகழும் – ஒளிபொருந்திய
பேர்தற்கு அகற்றுவதற்கு
திரியோகமருந்து – மூன்று யோகமருந்து
தெளிவு – நற்காட்சி
திறத்தன – தன்மை யுடையன
கூற்றவா – பிரிவுகளாக
பூணாய் – அணிகலன்களை அணிந்தவளே
பிணி – துன்பம்
ஓர்தல் – நல்லறிவு
பிறவார் – பிறக்கமாட்டார்


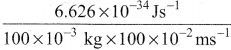

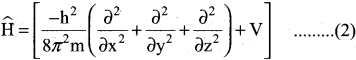

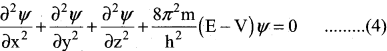
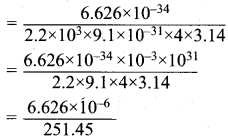
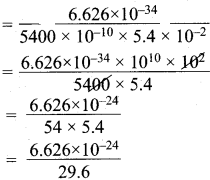

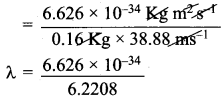
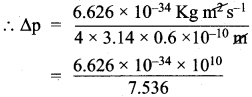
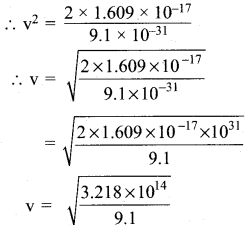
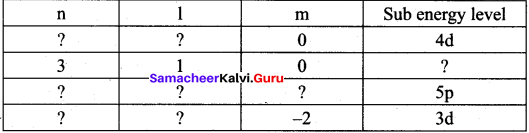
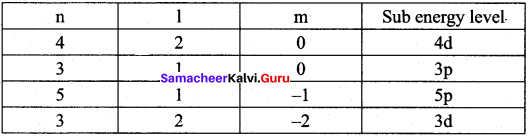
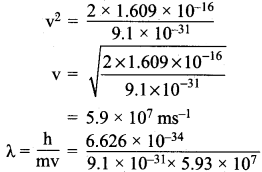
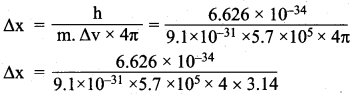




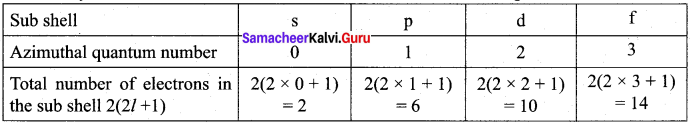
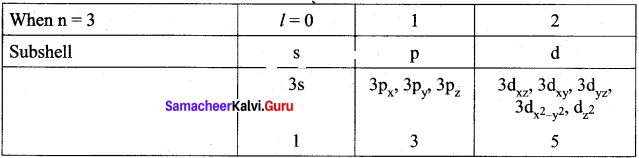
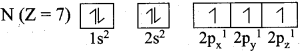
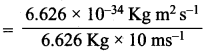
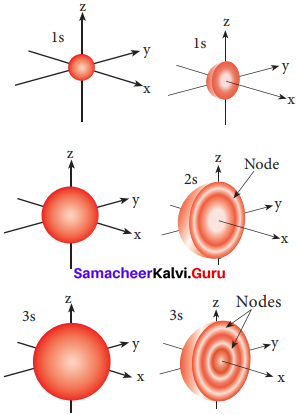
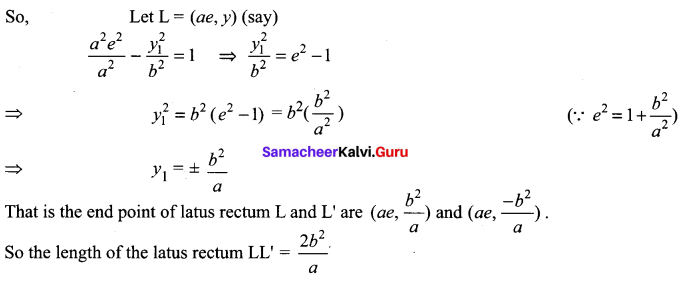
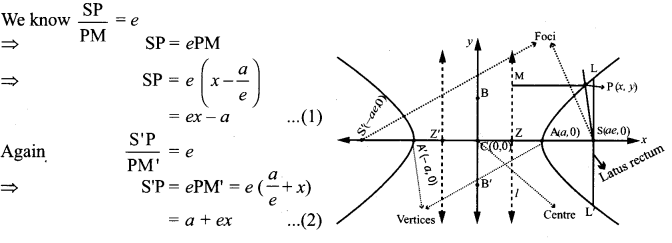
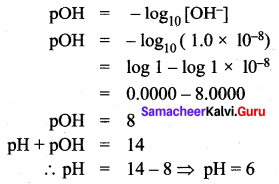
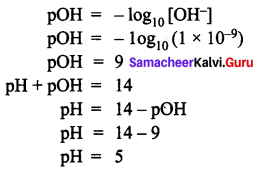
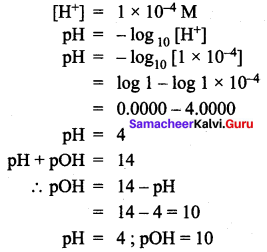
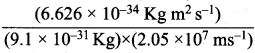 =355 x 10-4m.
=355 x 10-4m.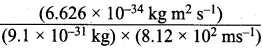
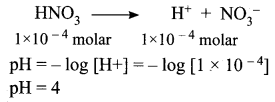
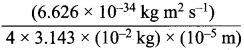 = 5.27 x 10-28mv
= 5.27 x 10-28mv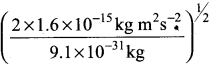 = 5.93 x 107m-1
= 5.93 x 107m-1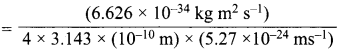 = 0.1 kg.
= 0.1 kg.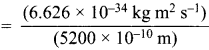
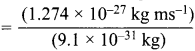 = 1.4 x 103 ms-1
= 1.4 x 103 ms-1