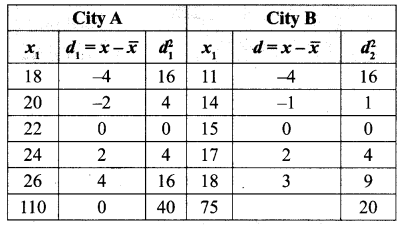
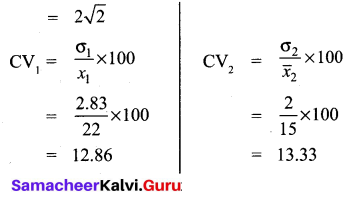
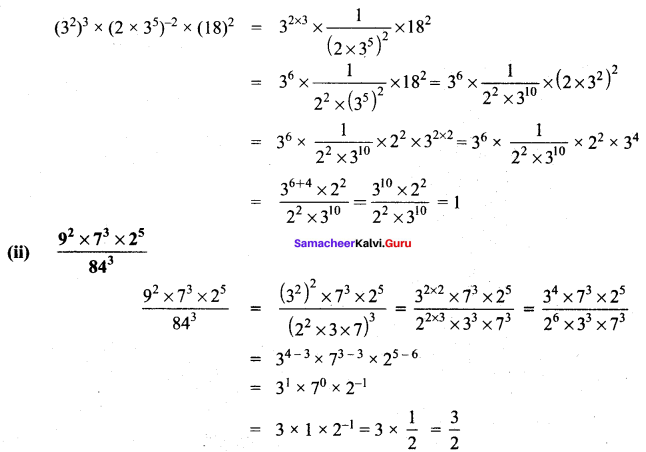
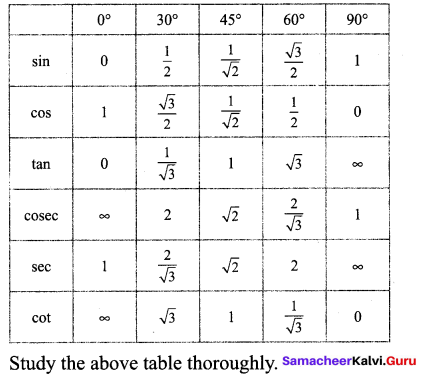
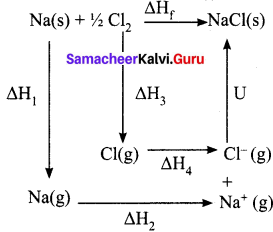
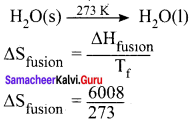
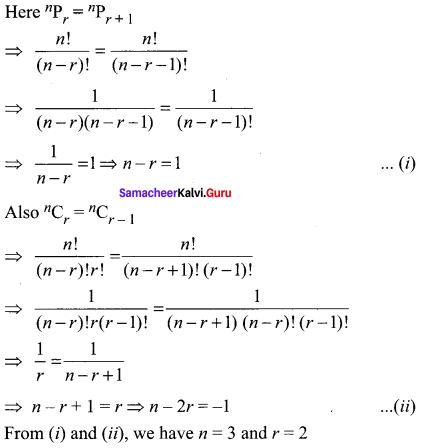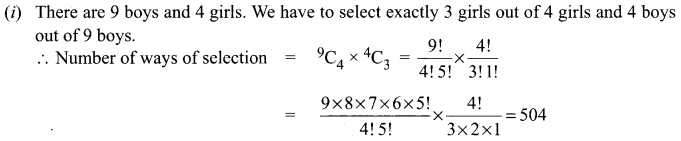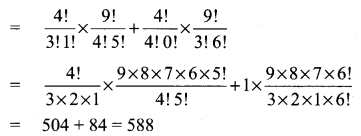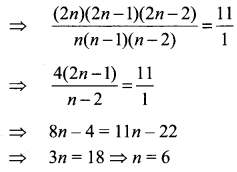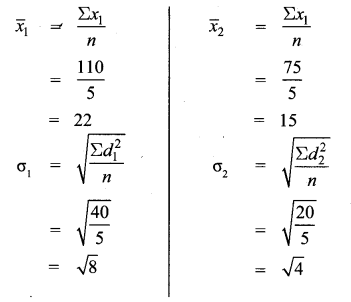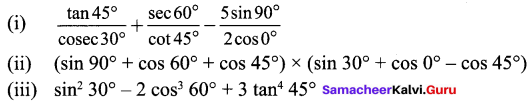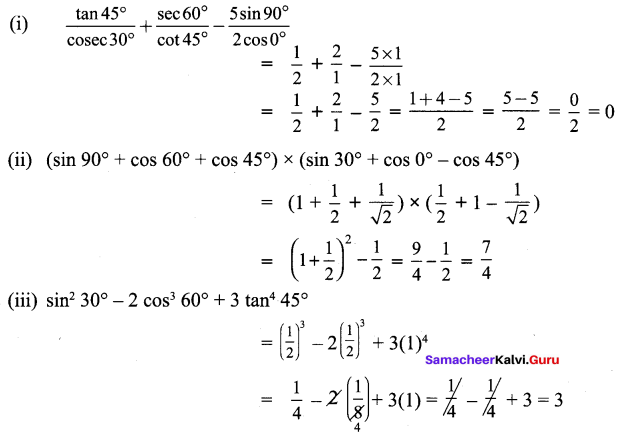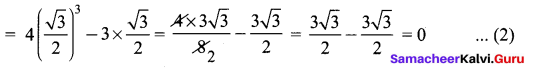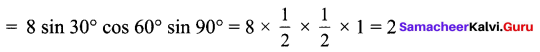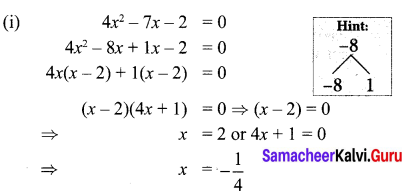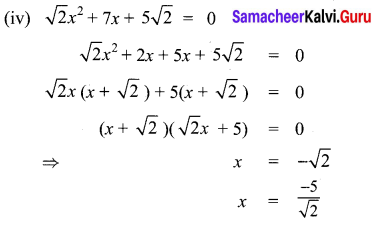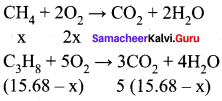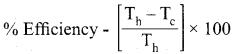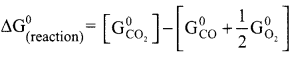Students can Download Maths Chapter 3 Algebra Ex 3.3 Questions and Answers, Notes Pdf, Samacheer Kalvi 8th Maths Book Solutions Guide Pdf helps you to revise the complete Tamilnadu State Board New Syllabus and score more marks in your examinations.
Tamilnadu Samacheer Kalvi 8th Maths Solutions Term 1 Chapter 3 Algebra Ex 3.3
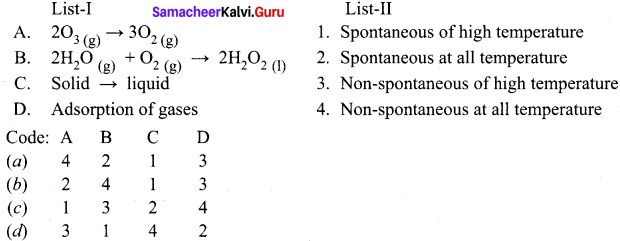
8th Maths Algebra Exercise 3.3 Question 1.
Expand
(i) (3m + 5)2
(ii) (5p – 1)2
(iii) (2n – 1)(2n + 3)
(iv) 4p2 – 25q2
Solution:
(i) (3m + 5)2
Comparing (3m + 5)2 with (a + b)2 we have a = 3m and b = 5
(a + b)2 = a2 + 2 ab + b2
(3m + 5)2 = (3m)2 + 2 (3m) (5) + 52
= 32m2 + 30m + 25 = 9m2 + 30m +25
(ii) (5p – 1)2
Comparing (5p – 1)2 with (a – b)2 we have a = 5p and b = 1
(a – b)2 = a2 – 2ab + b2
(5p – 1)2 = (5p)2 – 2 (5p) (1) + 12
= 52p2 – 10p + 1 = 25p2 – 10p + 1
(iii) (2n – 1)(2n + 3)
Comparing (2n – 1) (2n + 3) with (x + a) (x + b) we have a = -1; b = 3
(x + a) (x + b) = x2 + (a + b)x + ab
(2n +(- 1)) (2n + 3) = (2n)2 + (-1 + 3)2n + (-1) (3)
= 22n2 + 2 (2n) – 3 = 4n2 + 4n – 3
(iv) 4p2 – 25q2 = (2p)2 – (5q)2
Comparing (2p)2 – (5q)2 with a2 – b2 we have a = 2p and b = 5q
(a2 – b2) = (a + b)(a – b) = (2p + 5q) (2p – 5q)
Maths Chapter 3 Exercise 3.3 Class 8 Question 2.
Expand
(i) (3 + m)3
(ii) (2a + 5)3
(iii) (3p + 4q)3
(iv) (52)3
(v) (104)3
Solution:
(i) (3 + m)3
Comparing (3 + m)3 with (a + b)3 we have a = 3; b = m
(a + b)3 = a2 + 3a2b + 3 ab2 + b3
(3 + m)3 = 33 + 3(3)2 (m) + 3 (3) m2 + m3
= 27 + 27m + 9m2 + m3 = m3 + 9 m2 + 27m + 27
(ii) (2a + 5)3
Comparing (2a + 5)3 with (a + b)3 we have a = 2a, b = 5
(a + b)3 = a3 + 3a2b + 3ab2 + b3 = (2a)3 + 3(2a)2 5 + 3 (2a) 52 + 53
= 23a3 + 3(22a2) 5 + 6a (25) + 125
= 8a3+ 60a2 + 150a + 125
(iii) (3p + 4q)3
Comparing (3p + 4q)3 with (a + b)3 we have a = 3p and b = 4q
(a + b) 3 = a3 + 3a2b + 3ab2 + b3
(3p + 4q)3 = (3p)3 + 3(3p)2 (4q) + 3(3p)(4q)2 + (4q)3
= 33p3 +3 (9p2) (4q) + 9p (16q2) + 43q3
= 27p3 + 108p2q + 144pq2 + 64q3
(iv) (52)3 = (50 + 2)3
Comparing (50 + 2)3 with (a + b)3 we have a = 50 and b = 2
(a + b)3 = a3 + 3 a2b + 3 ab2 + b3
(50 + 2)3 = 503 + 3 (50)22 + 3 (50)(2)2 + 23
523 = 125000 + 6(2,500) + 150(4) + 8
= 1,25,000 + 15,000 + 600 + 8
523 = 1,40,608
(v) (104)3 = (100 + 4)3
Comparing (100 + 4)3 with (a + b)3 we have a = 100 and b = 4
(a + b)3 = a3 + 3 a2b + 3 ab2 + b3
(100 + 4)3 = (100)3 + 3 (100)2 (4) + 3 (100) (4)2 + (4)3
= 10,00,000 + 3(10000) 4 + 300 (16) + 64
= 10,00,000 + 1,20,000 + 4,800 + 64 = 11,24,864
Samacheer Kalvi 8 Maths Question 3.
Expand
(i) (5 – x)3
(ii) (2x – 4y)3
(iii) (ab – c)3
(iv) (48)3
(v) (97xy)3
Solution:
(i) (5 – x)3
Comparing (5 – x)3 with (a – b)3 we have a = 5 and b = x
(a – b)3 = a3 – 3a2b + 3ab2 – b3
(5 – x)3 = 53 – 3 (5)2 (x) + 3(5)(x2) – x3
= 125 – 3(25)(x) + 15x2 – x3 = 125
(ii) (2x – 4y)3
Comparing (2x – 4y)3 with (a – b)3 we have a = 2x and b = 4y
(a – b)3 = a3 – 3a2b + 3ab3 – b3
(2x – 4y)3 = (2x)3 – 3(2x)2 (4y) + 3(2x) (4y)2 – (4y)3
= 23x3 – 3(22x2) (4y) + 3(2x) (42y2) – (43y3)
= 8x3 – 48x2y + 96xy2 – 64y3
(iii) (ab – c)3
Comparing (ab – c)3 with (a – b)3 we have a = ab and b = c
(a – b)3 = a3 – 3a2b + 3ab2 – b3
(ab – c)3 = (ab)3 – 3 (ab)2 c + 3 ab (c)2 – c3
= a3b3 – 3(a2b2) c + 3abc2 – c3
= a3b3 – 3a2b2 c + 3abc2 – c3
(iv) (48)3 = (50 – 2)3
Comparing (50 – 2)3 with (a – b)3 we have a = 50 and b = 2
(a – b)3 = a3 – 3a2b + 3ab2 – b3
(50 – 2)3 = (50)3 – 3(50)2(2) + 3 (50)(2)2 – 23
= 1,25,000 – 15000 + 600 – 8 = 1,10,000 + 592
= 1,10,592
(v) (97xy)3
= 973 x3 y3 = (100 – 3)3 x3y3
Comparing (100 – 3)3 with (a – b)3 we have a = 100, b = 3
(a – b)3 = a3 – 3a2b + 3ab2 – b3
(100 – 3)3 = (100)3 – 3(100)2 (3) + 3 (100)(3)2 – 33
973 = 10,00,000 – 90000 + 2700 – 27
973 = 910000 + 2673
973 = 912673
97x3y3 = 912673x3y3
Samacheer Kalvi 8th Maths Book Question 4.
Simplify (i) (5y + 1)(5y + 2)(5y + 3)
(ii) (p – 2)(p + 1)(p – 4)
Solution:
(i) (5y + 1) (5y + 2) (5y + 3)
Comparing (5y + 1) (5y + 2) (5y + 3) with (x + a) (x + b) (x + c) we have x = 5y ; a = 1; b = 2 and c = 3.
(x + a) (x + b) (x + c) = x3 + (a + b + c) x2 (ab + bc + ca) x + abc
= (5y)3 + (1 + 2 + 3) (5y)2 + [(1) (2) + (2) (3) + (3) (1)] 5y + (1)(2) (3)
= 53y3 + 6(52y2) + (2 + 6 + 3)5y + 6
= 1253 + 150y2 + 55y + 6
(ii) (p – 2)(p + 1)(p – 4) = (p + (-2))0 +1)(p + (-4))
Comparing (p – 2) (p + 1) (p – 4) with (x + a) (x + b) (x + c) we have x = p ; a = -2; b = 1 ; c = -4.
(x + a) (x + b) (x + c) = x3 + (a + b + c) x2 + (ab + be + ca) x + abc
= p3 + (-2 + 1 + (-4))p2 + ((-2) (1) + (1) (-4) (-4) (-2)p + (-2) (1) (-4)
= p3 + (-5 )p2 + (-2 + (-4) + 8)p + 8
= p3 – 5p2 + 2p + 8
Samacheer Kalvi Guru 8th Maths Question 5.
Find the volume of the cube whose side is (x + 1) cm.
Solution:
Given side of the cube = (x + 1) cm
Volume of the cube = (side)3 cubic units = (x + 1)3 cm3
We have (a + b)3 = (a3 + 3a2b + 3ab2 + b3) cm3
(x + 1)3 = (x3 + 3x2 (1) + 3x (1)2 + 13) cm3
Volume = (x3 + 3x2 + 3x + 1) cm3
Samacheerkalvi.Guru 8th Maths Question 6.
Find the volume of the cuboid whose dimensions are (x + 2),(x – 1) and (x – 3).
Solution:
Given the dimensions of the cuboid as (x + 2), (x – 1) and (x – 3)
∴ Volume of the cuboid = (l × b × h) units3
= (x + 2) (x – 1) (x – 3) units3
We have (x + a) (x + b) (x + c) = x3 + (a + b + c) x2 + (ab + bc+ ca)x + abc
∴ (x + 2)(x – 1) (x – 3) = x3 + (2 – 1 – 3)x2 + (2 (-1) + (-1) (-3) + (-3) (2)) x + (2)(-1) (-3)
x3 – 2x2 + (-2 + 3 – 6)x + 6
Volume = x3 – 2x2 – 5x + 6 units3