Enhance your subject knowledge with Tamilnadu State Board Solutions for 24th Commerce Chapter 24 Types of Entrepreneurs Questions and Answers and learn all the underlying concepts easily. Make sure to learn the subject from Tamilnadu State Board Solutions Chapter 24 Types of Entrepreneurs Questions and Answers PDF on a day to day basis and score well in your exams. You can Download Samacheer Kalvi 24th Commerce Book Solutions Questions and Answers are given after enormous research by people having high subject knowledge and for better scoring grade. You can rely on them and prepare any topic of Commerce as per your convenience easily.
Tamilnadu Samacheer Kalvi 12th Commerce Solutions Chapter 24 Types of Entrepreneurs
Students those who are looking for Tamilnadu State Board Solutions Chapter 24 Types of Entrepreneurs Questions and Answers Concepts can find them all in one place from our site Tamilnadu State Board Solutions. Simply click on the links available to prepare the corresponding topics of Samacheer Kalvi 24th Commerce Book Solutions Questions and Answers easily. Clarify all your queries from chapter wise different questions to be familiar with the kind of questions appearing in the exam. Thus, you can increase your score and get higher grade in the final exam.
Samacheer Kalvi 12th Commerce Types of Entrepreneurs Textbook Exercise Questions and Answers
I. Choose the Correct Answer
Question 1.
Choose the type of entrepreneur that isn’t based on function:
(a) Innovative
(b) Classical
(c) Fabian
(d) Drone
Answer:
(c) Fabian
Question 2.
Choose the type of Entrepreneur that is not based on Motivation:
(a) Pure
(b) Corporate
(c) Spontaneous
(d) Induced
Answer:
(c) Spontaneous
Question 3.
Which of the following is the Activity of a Business Entrepreneur?
(a) Production
(b) Marketing
(c) Operation
(d) All of the above
Answer:
(d) All of the above
Question 4.
Find the odd one out in context of Trading Entrepreneur.
(a) Selling
(b) Commission
(c) Buying
(d) Manufacturing
Answer:
(d) Manufacturing
Question 5.
Corporate Entrepreneur is also called as _________
(a) Intrapreneur
(b) Promoter
(c) Manager
(d) Shareholder
Answer:
(b) Promoter
Question 6.
Poultry, Flowers, Fruits, etc., are called allied products of _________ entrepreneur.
(a) Corporate
(b) Retail
(c) Trading
(d) Agricultural
Answer:
(d) Agricultural
Question 7.
_________ Entrepreneur Supply Services Unlike.
(a) Hoteliers
(b) Banking
(c) Airlines
(d) Livestock
Answer:
(d) Livestock
Question 8.
Motive of a Pure Entrepreneur is _________
(a) Rendering service
(b) Earning profit
(c) Attaining status
(d) Both b and c
Answer:
(d) Both b and c
Question 9.
Which of these is based on Technology?
(a) Modem
(b) Professional
(c) Corporate
(d) Industrial
Answer:
(c) Corporate
Question 10.
Which of the below is not a characteristic of a Fabian Entrepreneur?
(a) Conservative
(b) Risk averse
(c) Sceptical
(d) Adaptive
Answer:
(d) Adaptive
II. Very Short Answer Questions
Question 1.
What is the other name of business entrepreneur?
Answer:
The term entrepreneur means the. person who takes steps for commencing the business. So he is otherwise called as organiser or proprietor.
Question 2.
Mention the other name for corporate entrepreneur.
Answer:
Corporate entrepreneur is called promoter. He/she takes initiative necessary to start an entity under corporate format.
Question 3.
Who are agricultural entrepreneur?
Answer:
Agricultural entrepreneurs are those entrepreneurs who raise farm products and market them.
Question 4.
State the name of the.following ventures:
a. Started by individuals for profit motive
b. Started by Government
c. Started by individuals and Government together
d. Started as a family business
Answer:
a. Private entrepreneur
b. State entrepreneurship
c. Induced entrepreneur
d. Classical entrepreneur
Question 5.
Give some examples of pure entrepreneurs.
Answer:
Dhirubai Ambani, Jamshedji Tata, T.V. Sundaram Iyengar, Seshadriji, Birla, Narayanamurthi, and Azim Premji are few examples of pure entrepreneurship.
III. Short Answer Questions
Question 1.
Who is a private entrepreneur?
Answer:
Ventures started by individual either singly or collectively at their own risk after mobilising – various, resources in order to earn profit are called private entrepreneurship.
Question 2.
What is political environment?
Answer:
To commence business, various factors are needed. Apart from all other factors, political environment is also essential. It means that the concessions, incentives provided by the government drive them to enter into venture. The government also provides support in the form of loans, subsidies and other taxes.
Question 3.
List down few examples of pure entrepreneurship.
Answer:
Pure entrepreneurs are individuals who are propelled to enter into venture by psychological and economic motives. They nurture desire of starting a particular venture and earning high profit there from and thus attaining a social status. They apply their knowledge, skill and insight in making the venture a great’ success in order to earn maximum profit out of the venture. Dhirubai Ambani, Jamshedji Tata, T.V. Sundaram Iyengar, Seshadriji, Birla, Narayanamurthi, and Azim Premji are few examples of pure entrepreneurship.
Question 4.
How does a professional entrepreneur operate?
Answer:
Professional entrepreneur is one who is having a rich expertise in starting a venture but lack interest in continuing the venture as a manager or as a owner. He/she simply sells out the venture started by him to someone else after its successful take-off.
Question 5.
Explain, about the agricultural entrepreneur.
Answer:
Agricultural entrepreneurs are those entrepreneurs who raise farm products and market them. They use the various inputs like labour, fertilizer, insecticide, water technology, etc., to raise the products and market their products either directly or through co-operative entities or through brokers or through tie up with large retailers.
IV. Long Answer Questions
Question 1.
Explain in detail on classification according to the type of business.
Answer:
Classification of Entrepreneur according to the type of business:
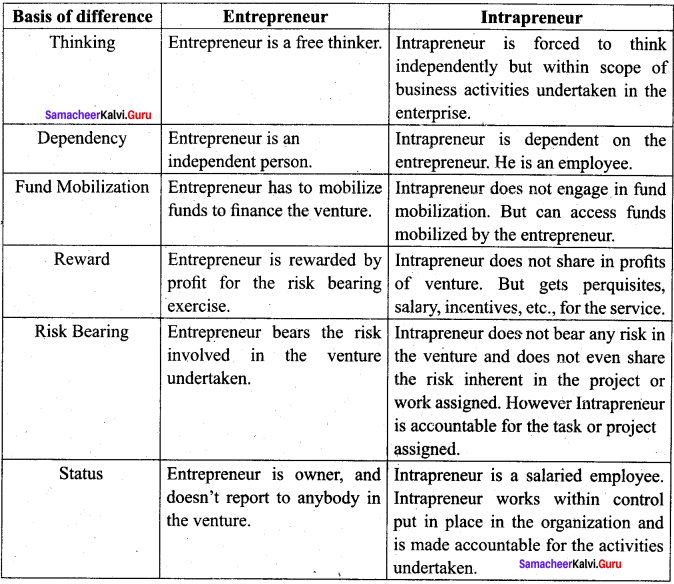
(i) Business Entrepreneur: He is called solo entrepreneur. He is the one who finds out an idea for a new product or service and establish a business enterprise.
(ii) Trading Entrepreneur: Trading entrepreneurs are those who restrict themselves to buying and selling finished goods.
(iii) Industrial Entrepreneur: These are entrepreneurs who manufacture products to cater to
(iv) Corporate Entrepreneur: He is called as promoter. He takes initiative necessary to start an entity under corporate format.
(v) Agricultural Entrepreneur: These entrepreneurs are those who raise farm products and market them.
Question 2.
Discuss the nature of functional entrepreneurs.
Answer:
Nature of functional entrepreneurs:
(i) Innovative Entrepreneur: He is a person who introduces new project. They observe the environment regarding information to the new venture.
(ii) Imitative Entrepreneur: He refers to the person who simply imitates existing knowledge or technology already in advance countries. Redesigning of products suited to the local conditions.
(iii) Fabian Entrepreneur: These are said to be conservatives and sceptical about any changes in their organisation. They are of risk-averse.
(iv) Drone Entrepreneur: Drone entrepreneurs are those who are totally opposed to changes in the environment. They used to operate in the-niche market.
Question 3.
Distinguish between the rural and urban entrepreneur.
Answer:
| S. No. | Rural Entrepreneur | Urban Entrepreneur |
| 1. | It refers to the person who starts business in rural areas. | It refers to the person who commences business in urban areas. |
| 2. | These entrepreneurs start doing business in the villages and small towns. | They will do their business in state capital, towns, district headquarters, municipalities, etc. |
| 3. | They may be agricultural and trading entrepreneurs. | They may be industrial or corporate entrepreneur. |
| 4. | The availability of material and labour is easy. So the cost of operation tends to be low. | The availability of material and labour may be difficult. So the cost of operation may be high. |
Samacheer Kalvi 12th Commerce Types of Entrepreneurs Additional Questions and Answers
I. A. Choose the Correct Answer
Question 1.
The entrepreneurs classified on the basis of type of business are __________
(i) Industrial entrepreneur
(ii) Technical entrepreneur
(iii) Professional entrepreneur
(iv) Business entrepreneur
(a) (i) and (iii)
(b) (i) and (iv)
(c) (ii) and (iii)
(d) (ii) and (iv)
Answer:
(b) (i) and (iv)
B. Fill in the blanks
Question 1.
Entrepreneur who commences his entrepreneurial activity in urban areas is called as __________
Answer:
urban entrepreneur
II. Very Short Answer Questions
Question 1.
Who is a service entrepreneur?
Answer:
The entrepreneurs enter into the business of giving service products to end consumers. Example: Banking, Insurance and Transport services.
Question 2.
Write a short note on retail entrepreneurs.
Answer
Retail entrepreneurs are those who enter into venture of distributing the end product to final consumer. They used to buy the goods from numerous wholesalers.
III. Short Answer Questions
Question 1.
Who are industrial entrepreneurs?
Answer:
Industrial entrepreneurs are those persons who manufacture products to cater the needs of the consumers. They may be small, medium and large entrepreneurs. Industrial entrepreneurs mobilise the resources of various types’.
Question 2.
Who are technical entrepreneurs?
Answer:
Technical entrepreneurs are those craftsmen like welder, fitter, turner, carpenter and goldsmith, photographer, weaver who start small business. They manufacture products/service of high quality. They simply focus on production rather than on marketing.
We as a team believe the information prevailing regarding the Tamilnadu State Board Solutions for 24th Commerce Chapter 24 Types of Entrepreneurs Questions and Answers has been helpful in clearing your doubts to the fullest. For any other help do leave us your suggestions and we will look into them. Stay in touch to get the latest updates on Tamilnadu State Board Solutions for different subjects in the blink of an eye.
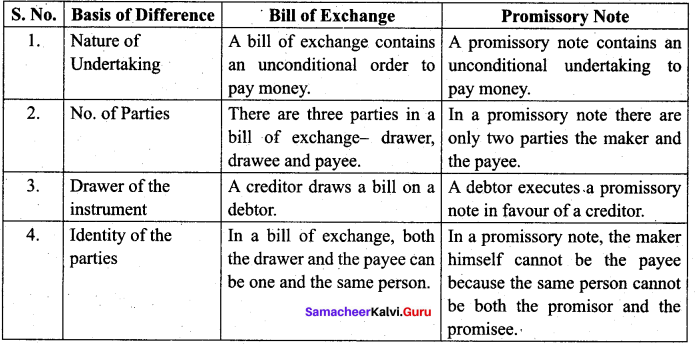
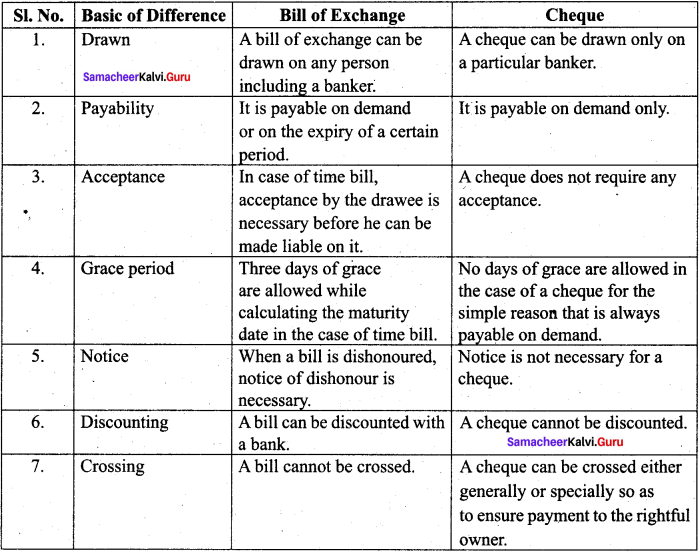
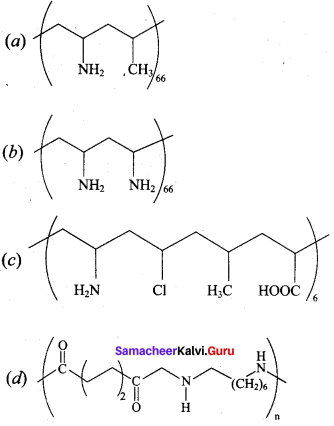
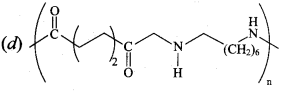
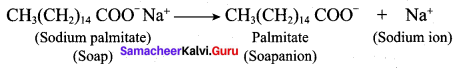
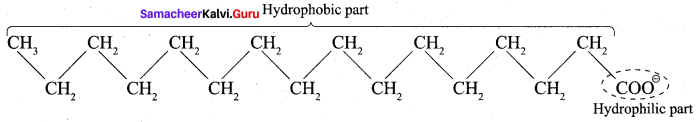
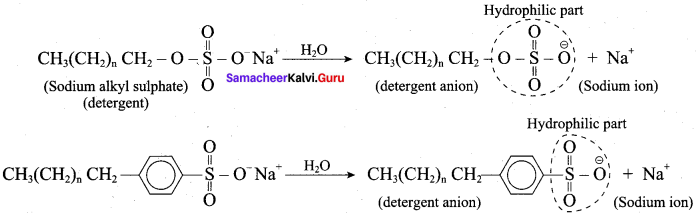
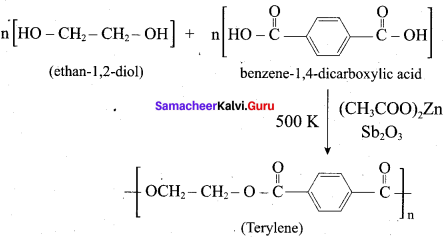
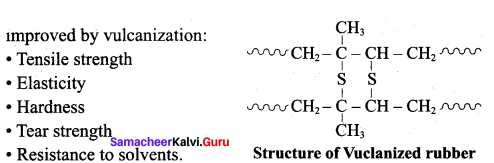
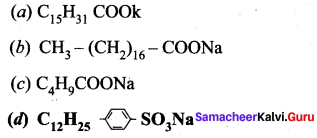
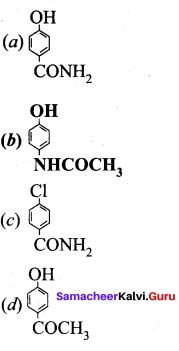
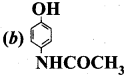
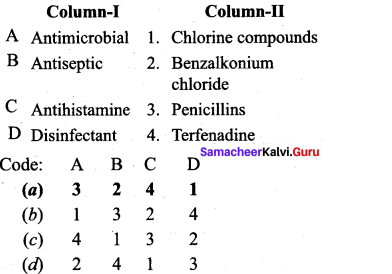
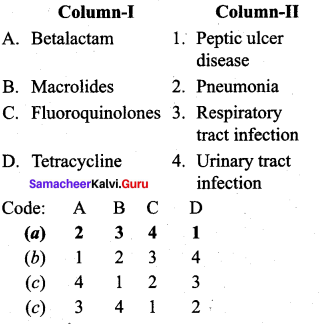
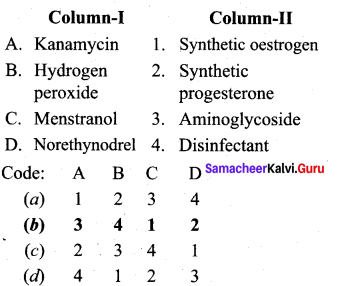
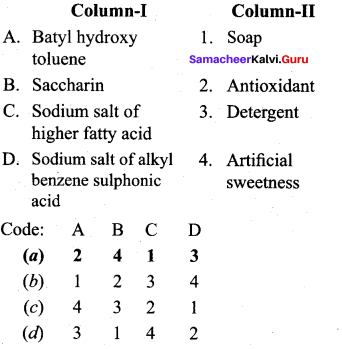
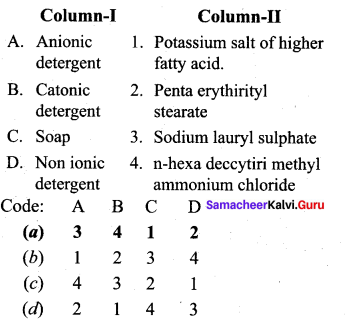
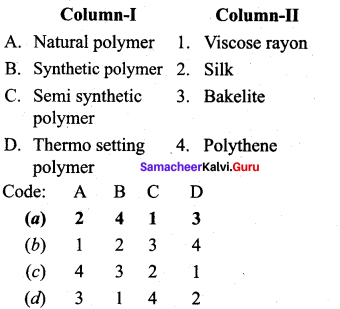
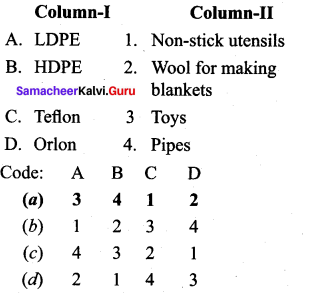
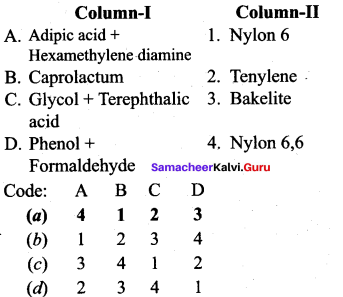
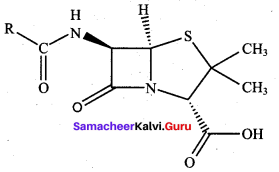
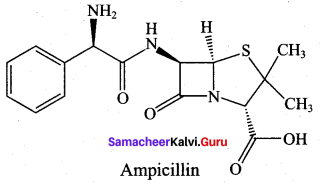
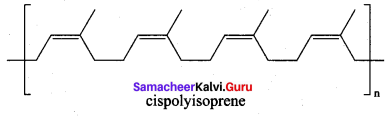
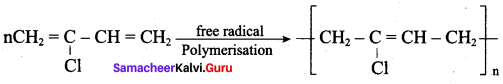

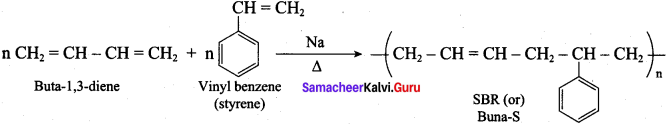
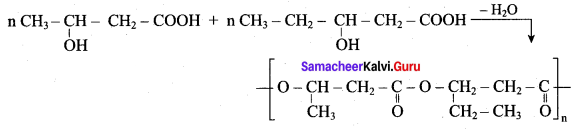

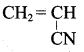
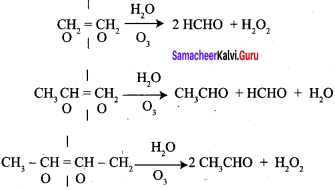
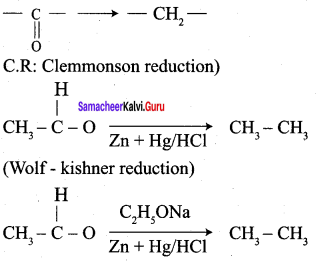
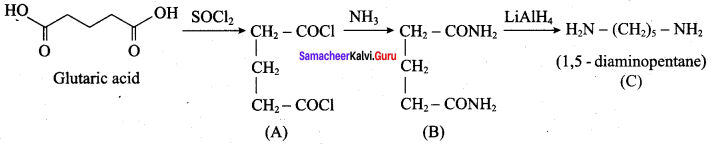
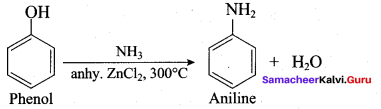
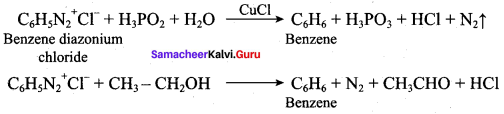
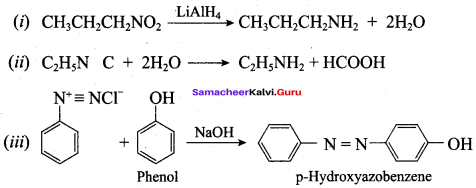
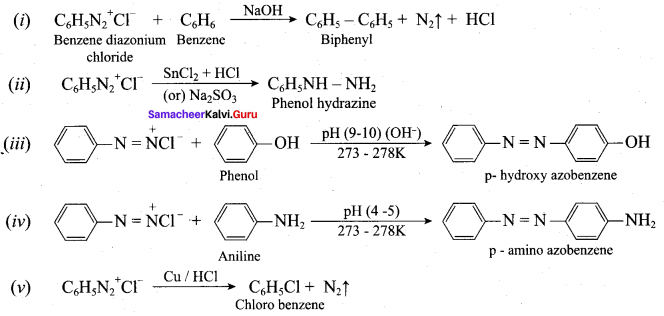
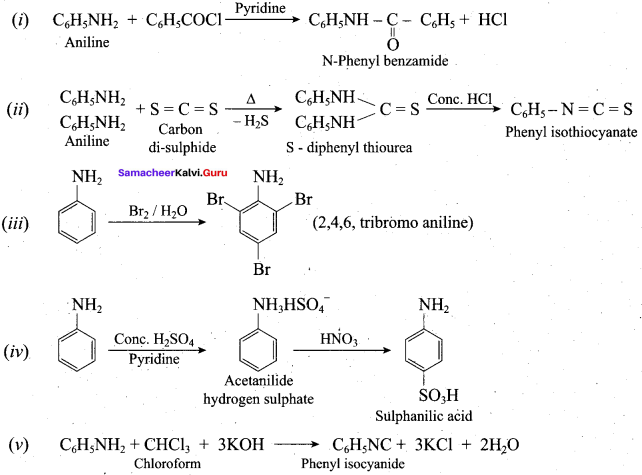
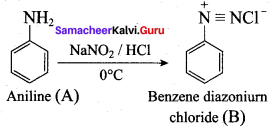
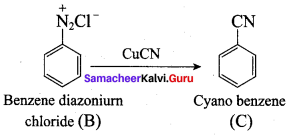
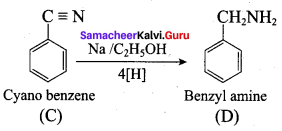
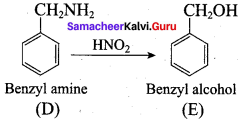
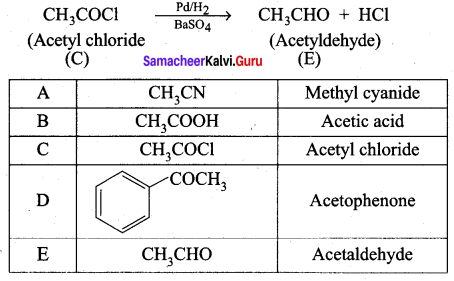
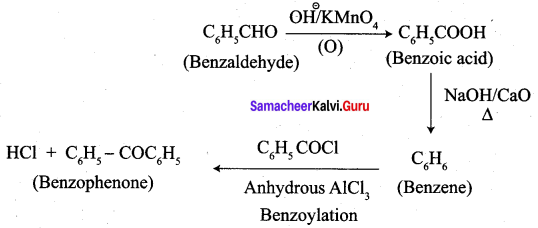
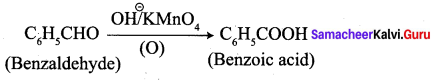
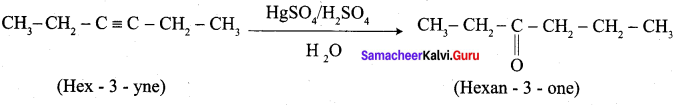
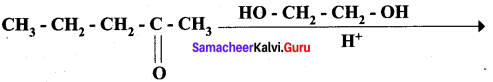
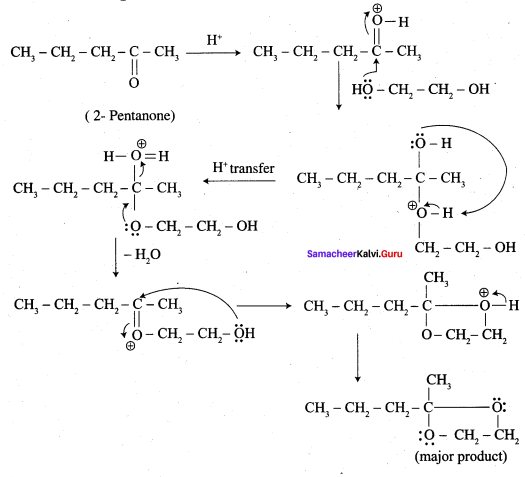
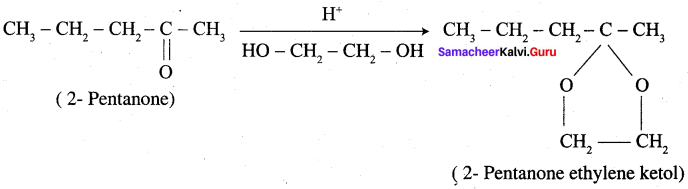
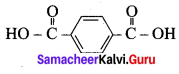 ; Ethylene glycol (Ethane – 1, 2 – diol)
; Ethylene glycol (Ethane – 1, 2 – diol)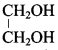
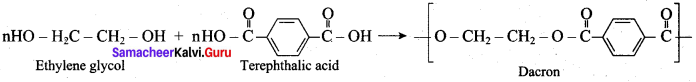

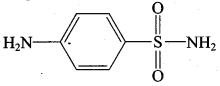

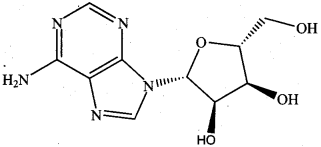
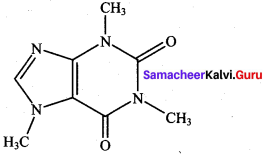
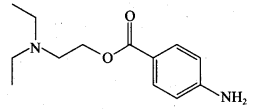


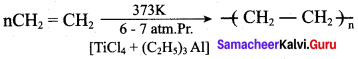
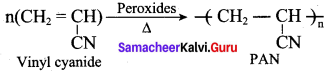
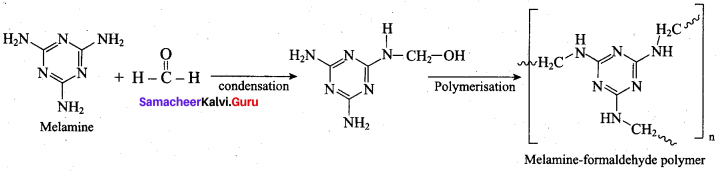
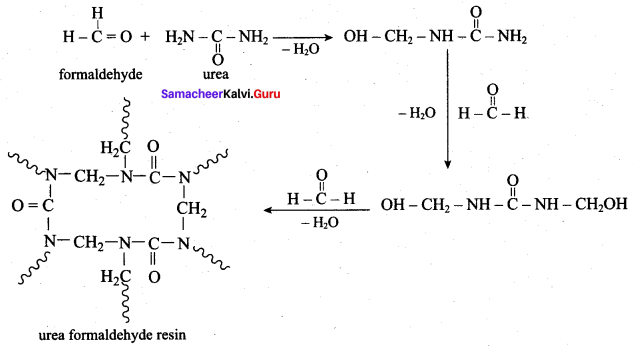
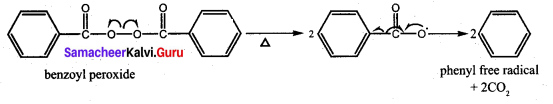
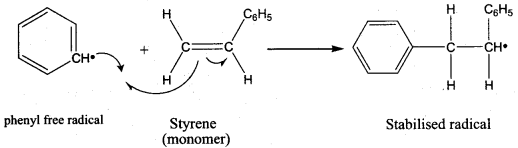
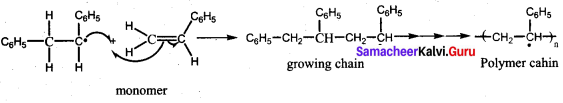
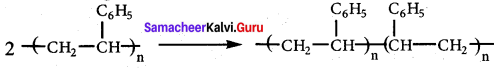
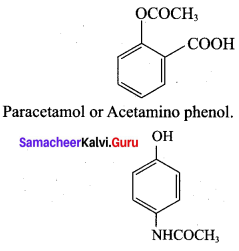
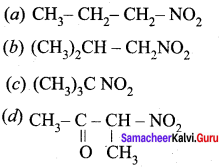
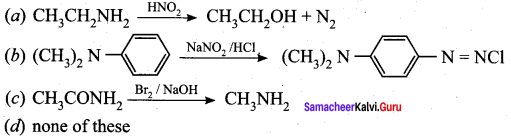
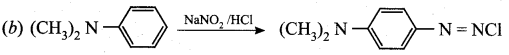
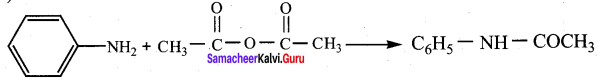
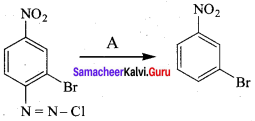
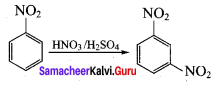

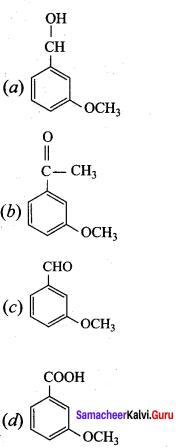
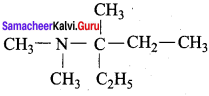 is ………………
is ………………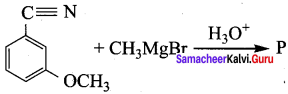
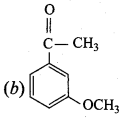
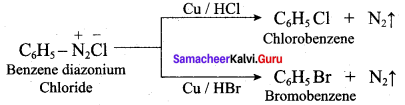
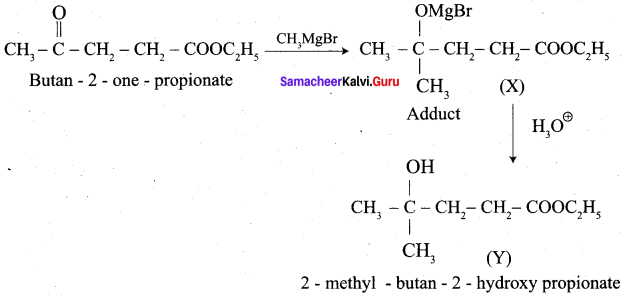
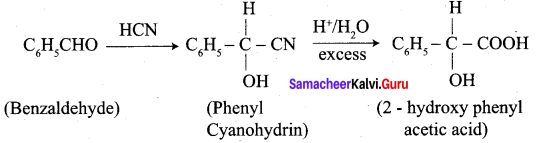
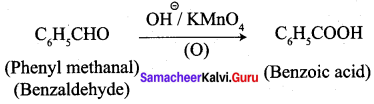
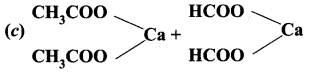
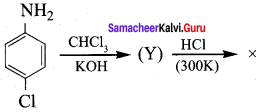 + Methanoic acid
+ Methanoic acid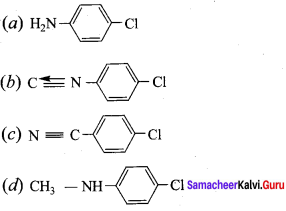
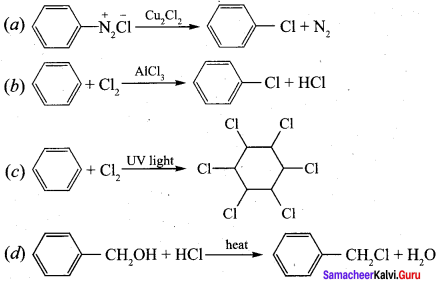
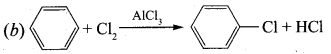
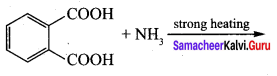
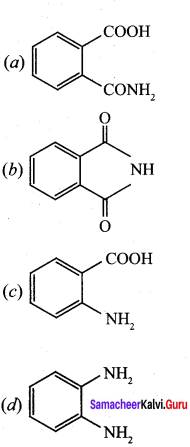
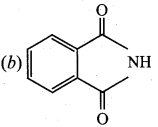
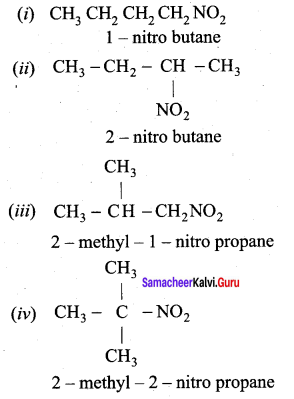
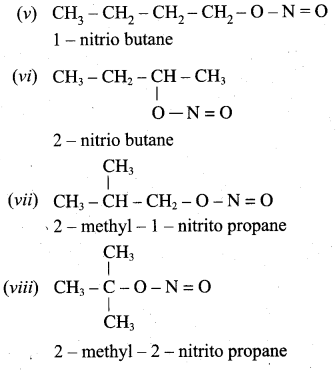
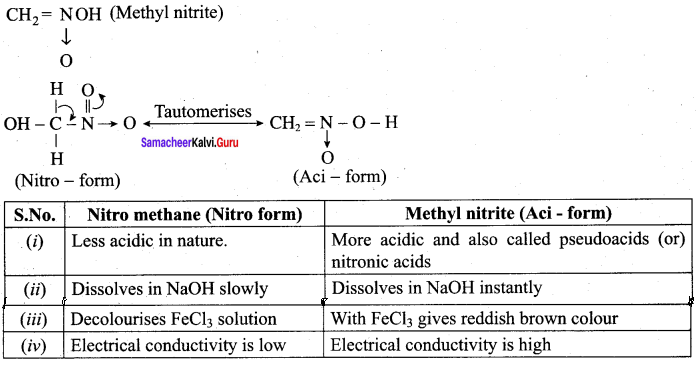
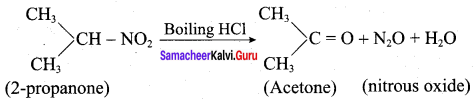
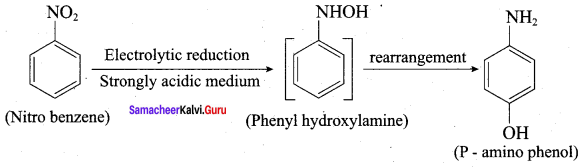
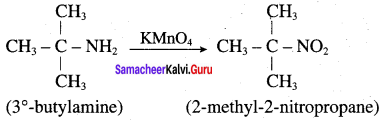
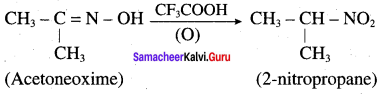
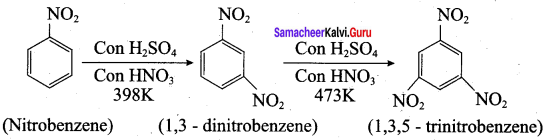
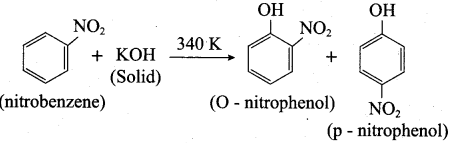
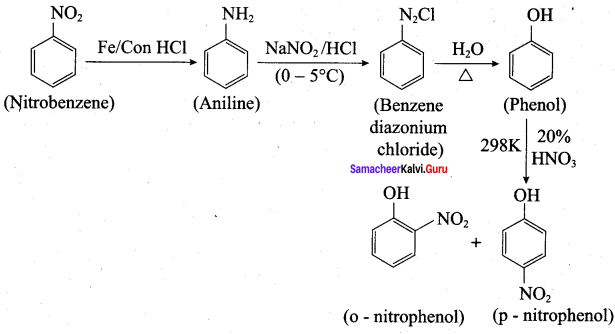
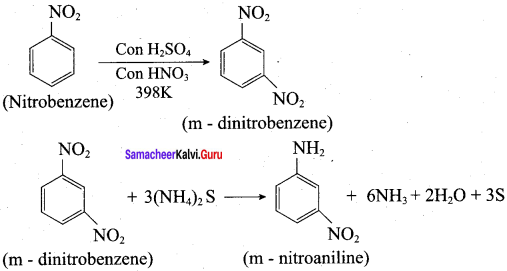
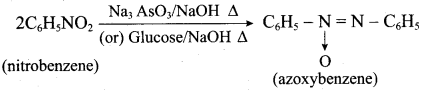
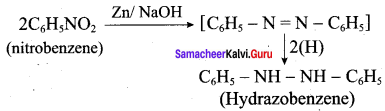
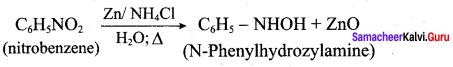
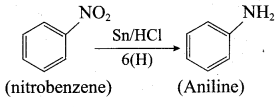
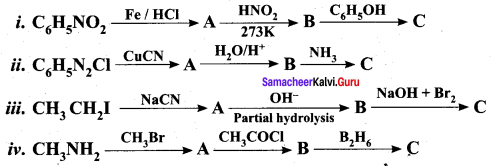
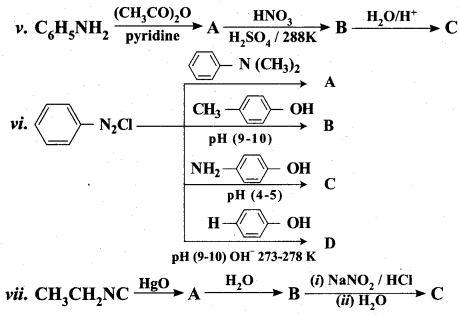
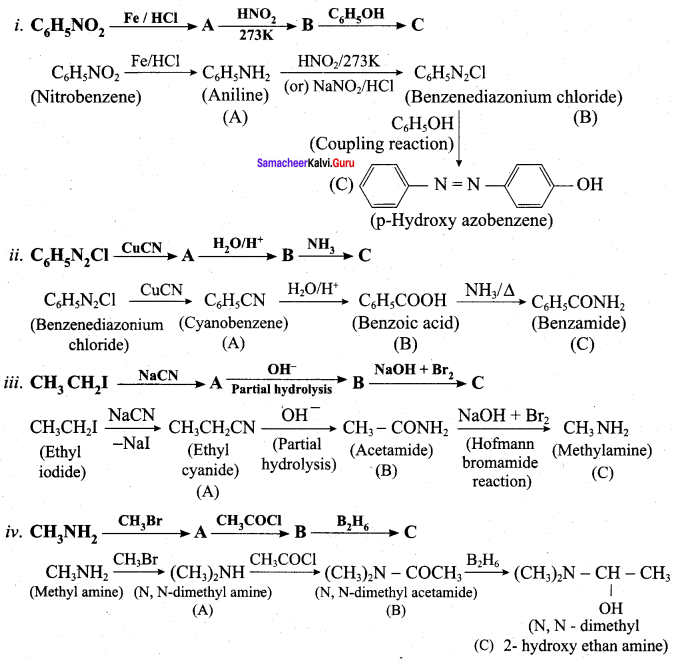
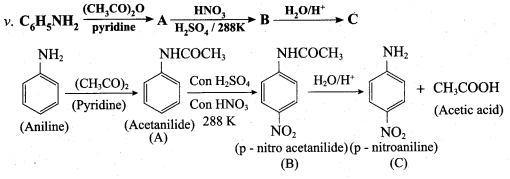
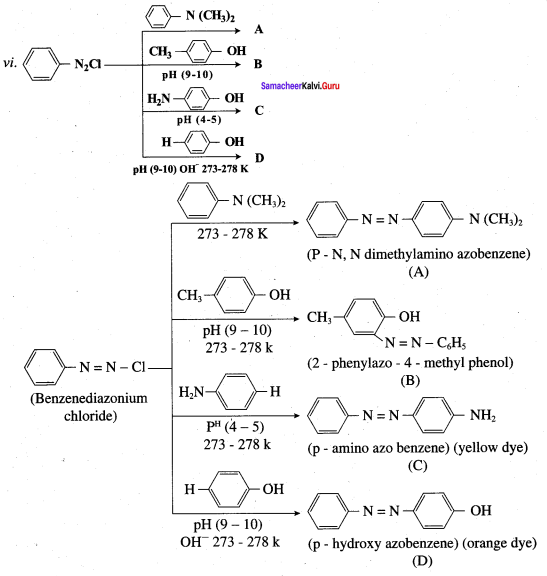
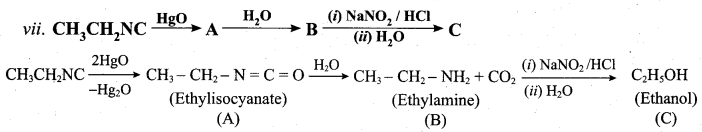
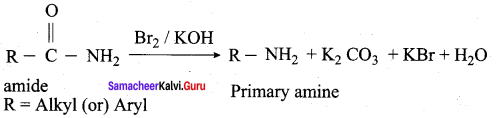
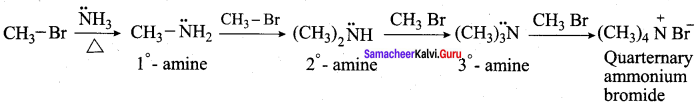
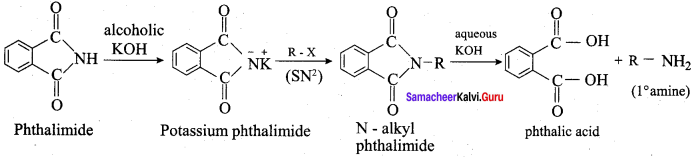
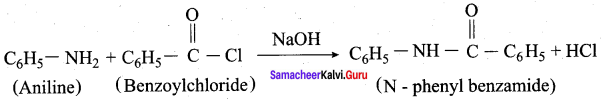
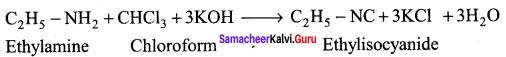
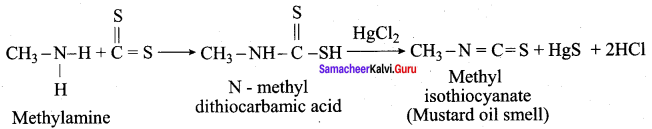
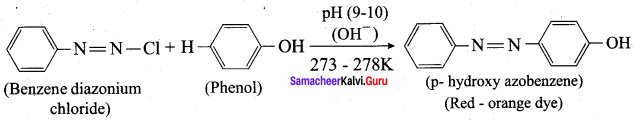
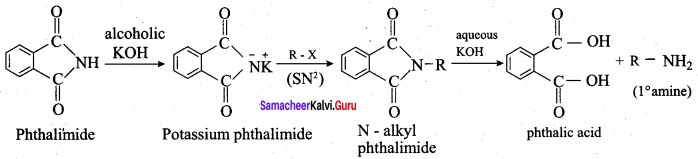
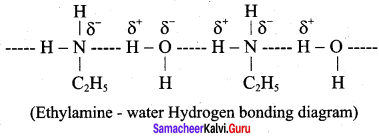
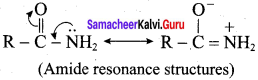
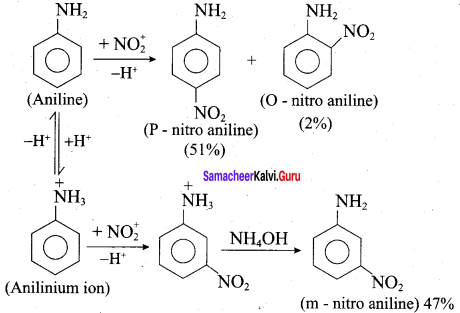
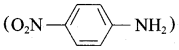
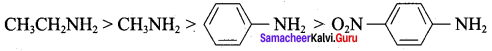
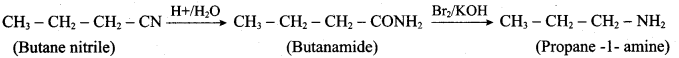
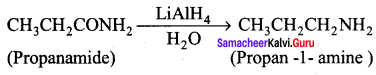
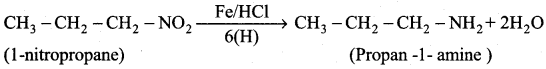


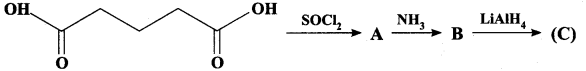
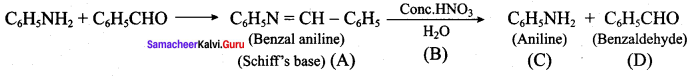
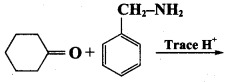
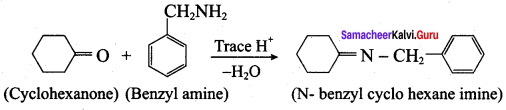
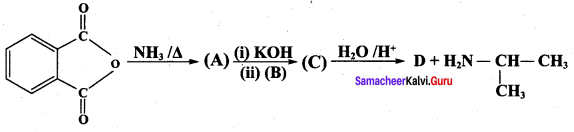
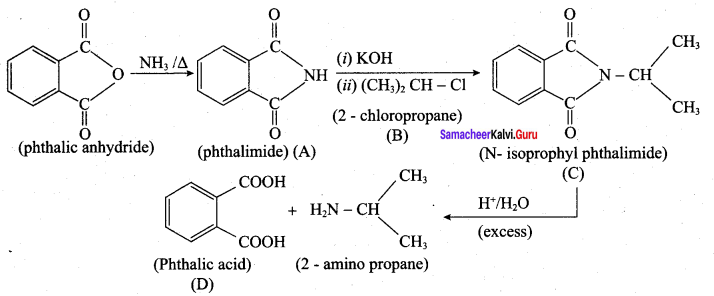
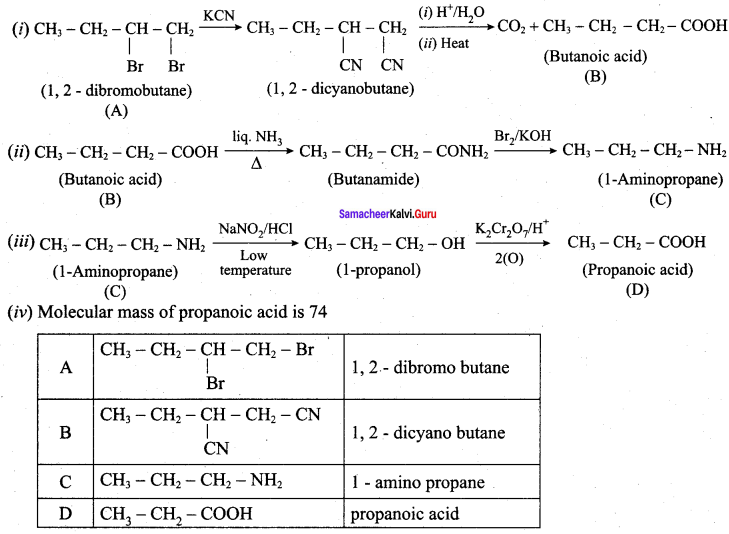
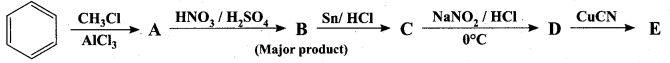
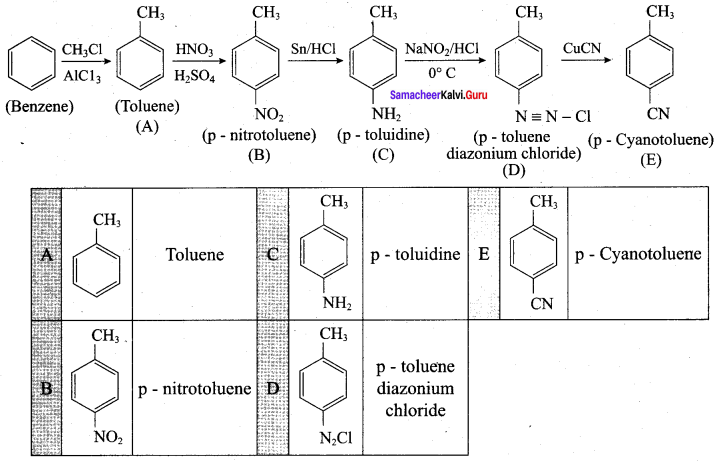
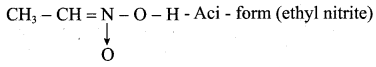

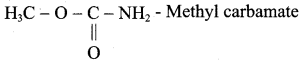
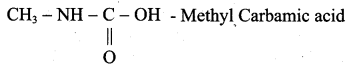
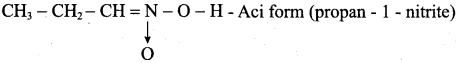


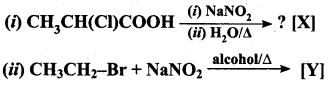
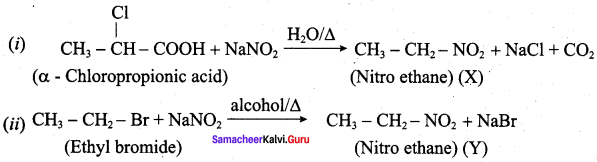
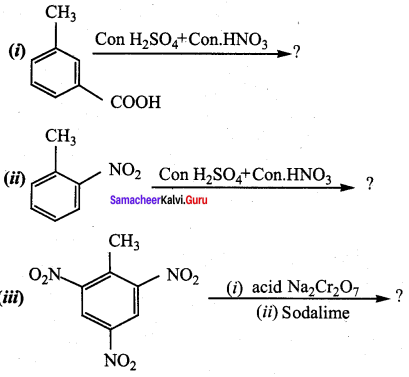
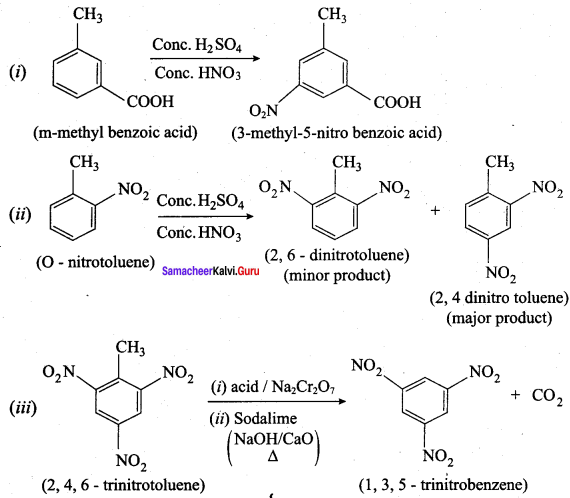
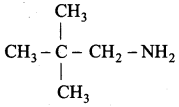
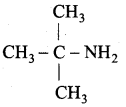
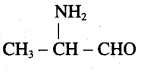
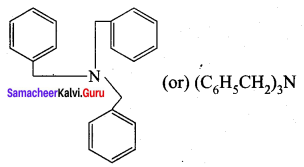
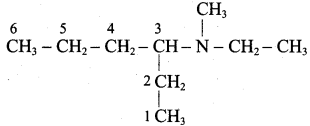
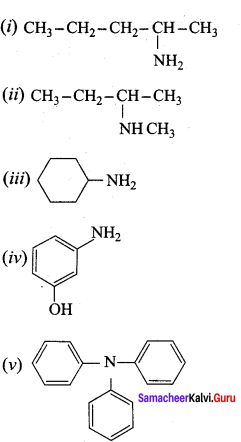
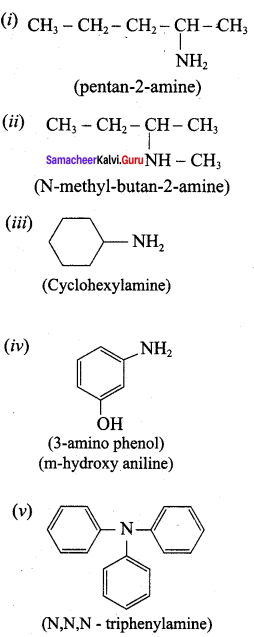
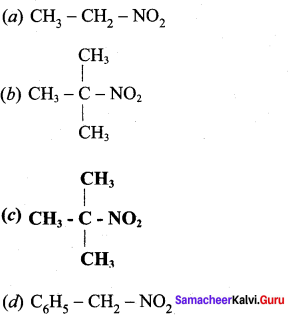
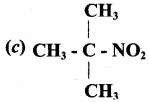
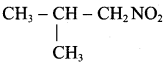 is ………………
is ………………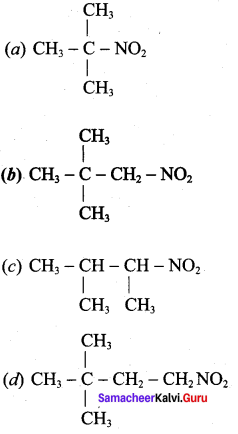
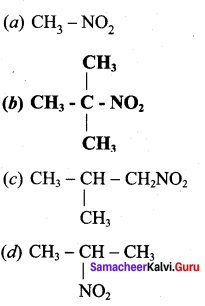
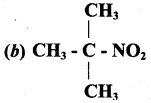
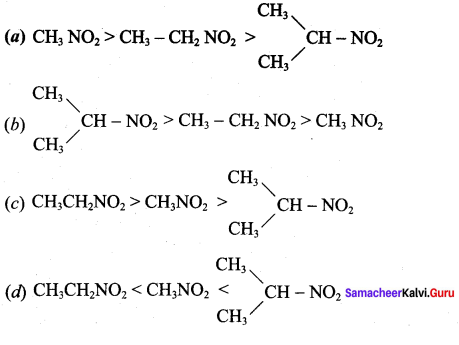
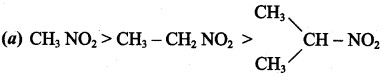
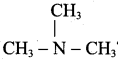
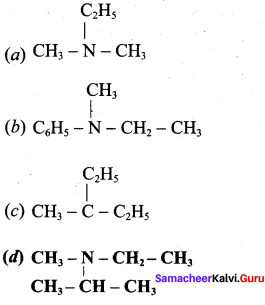
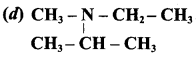
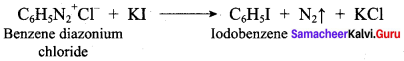
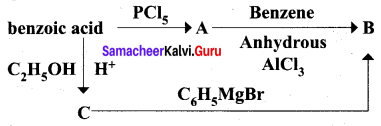
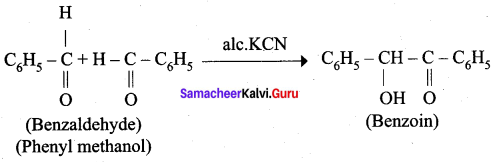
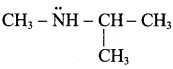 is ……………..
is ……………..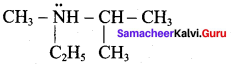
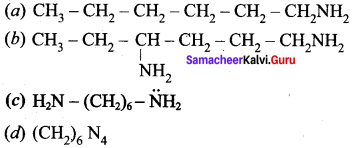
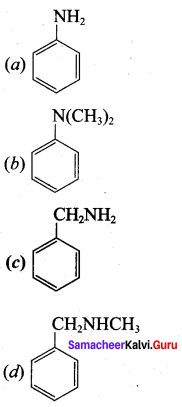
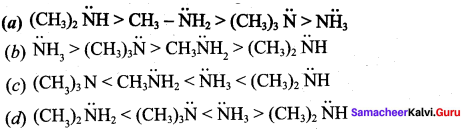
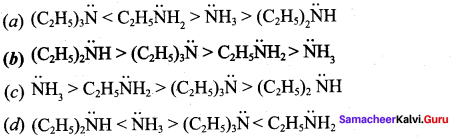
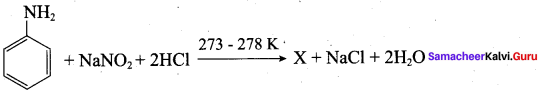
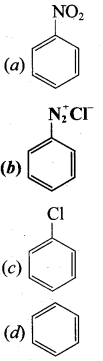
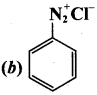
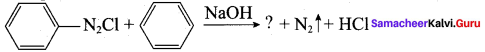
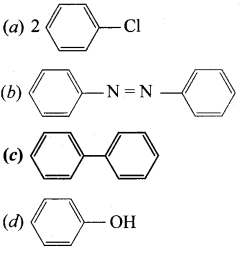
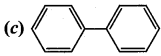
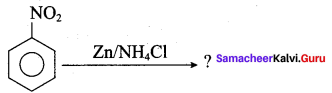
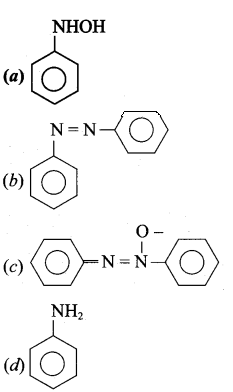
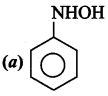
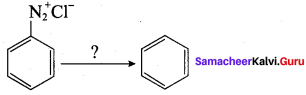
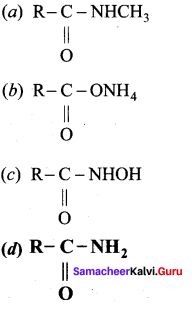
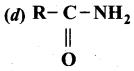
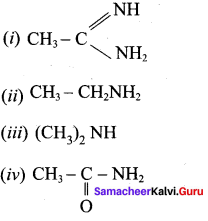
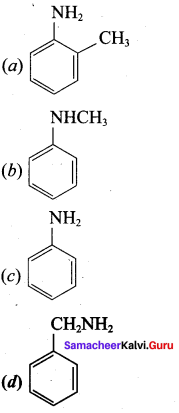
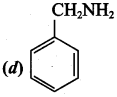
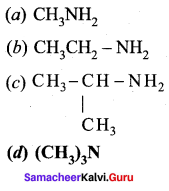
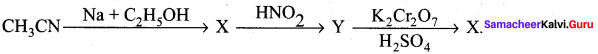

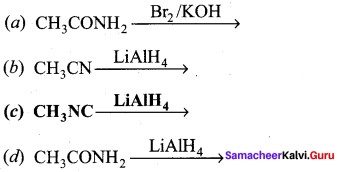
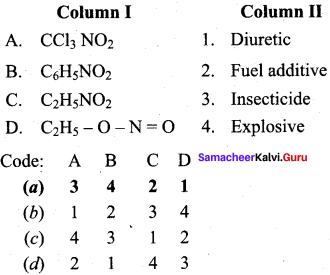
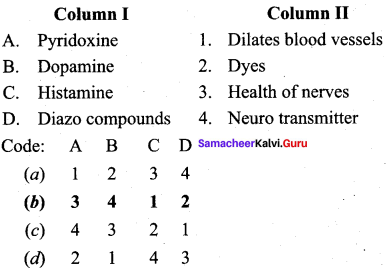
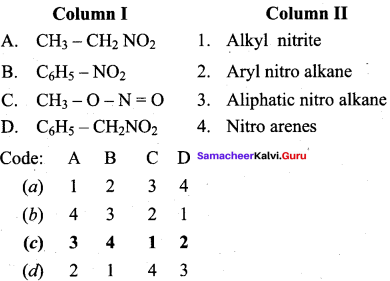
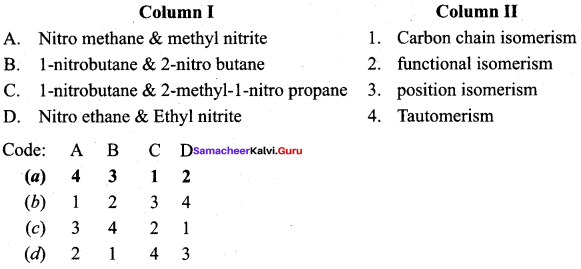
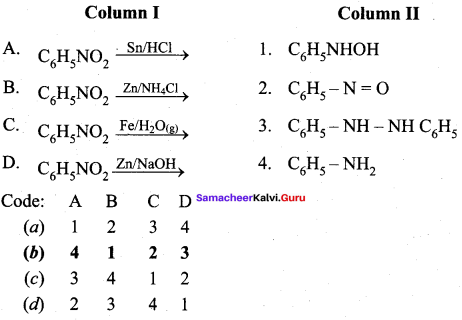
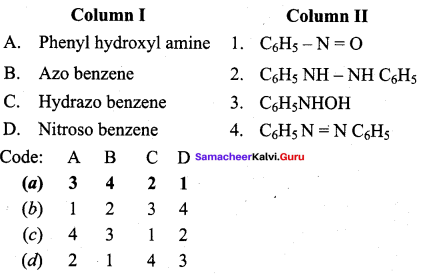
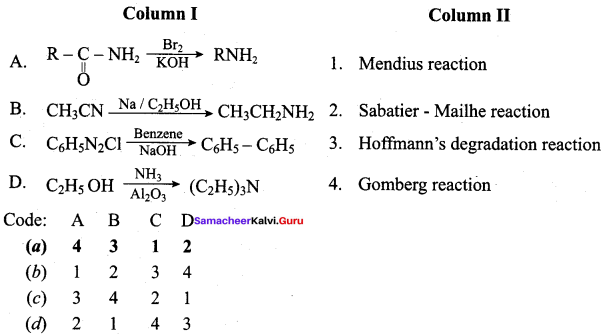
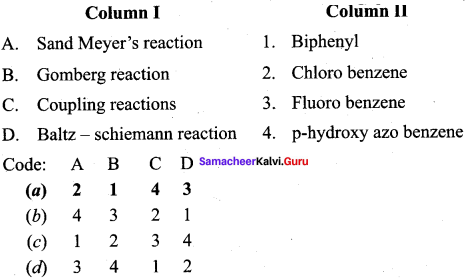
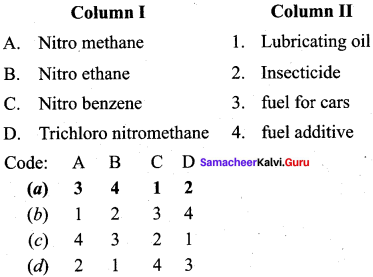
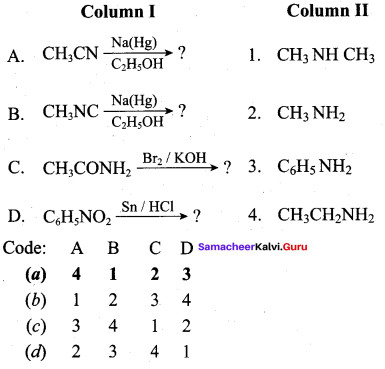

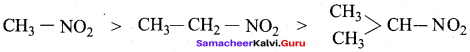
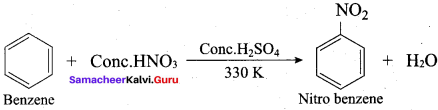
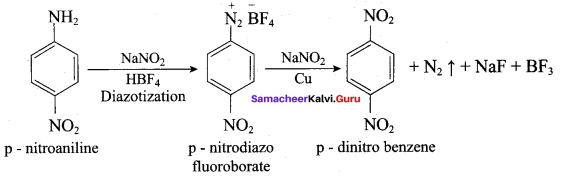
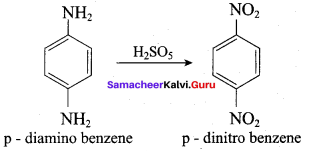
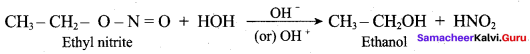
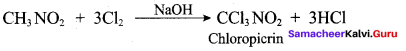

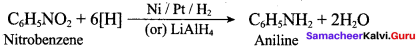
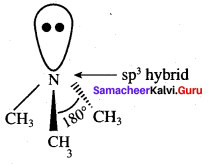
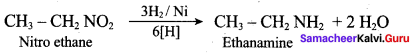
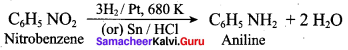
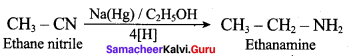
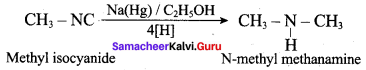
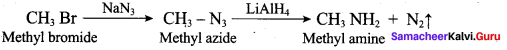
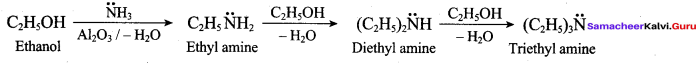
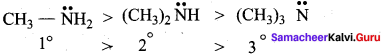

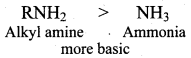
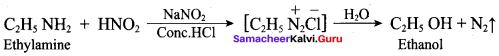
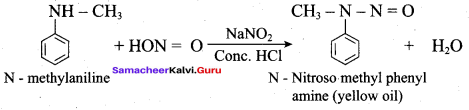
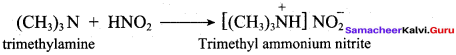
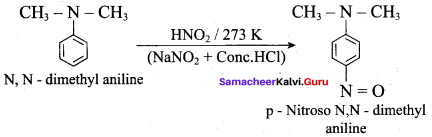
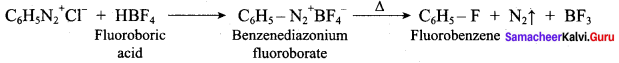
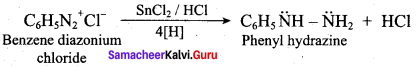
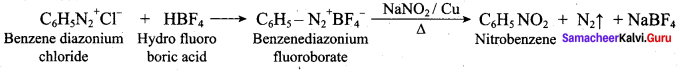
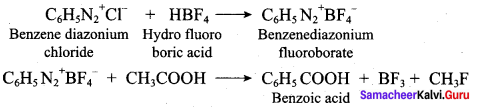
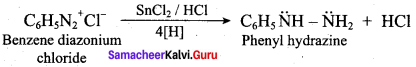

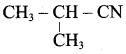
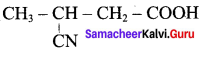
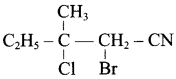
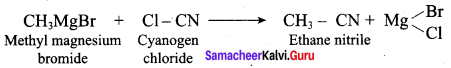
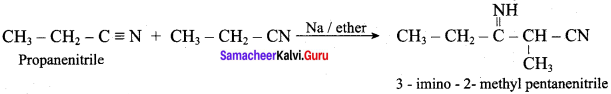
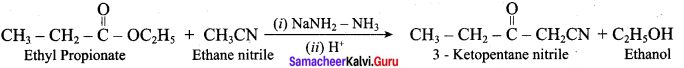
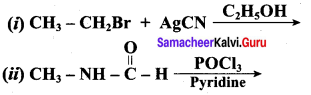
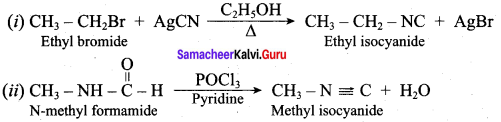
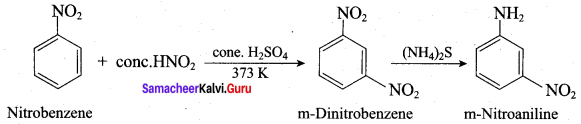
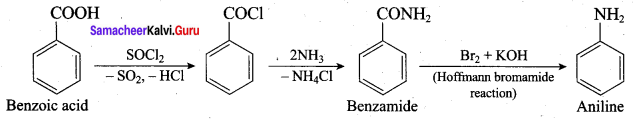
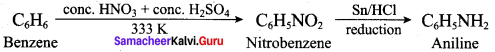
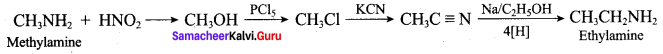
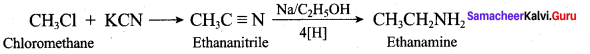
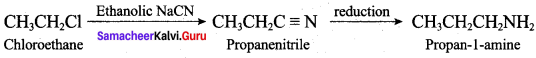
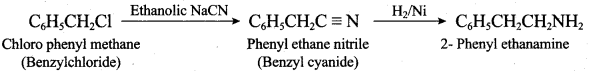
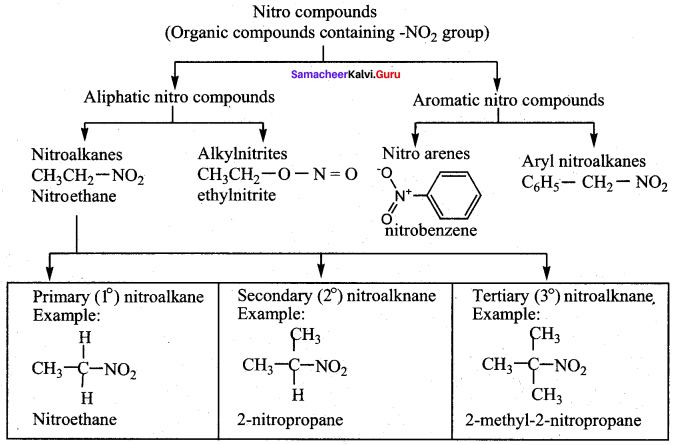
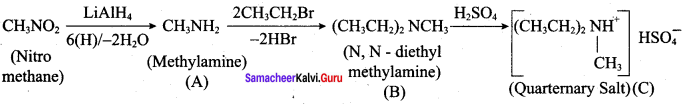
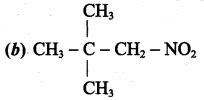
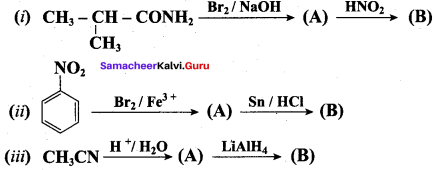
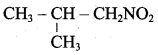 2 – methyl – 1 – nitropropane
2 – methyl – 1 – nitropropane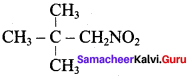 2, 2 – dimethyl – 1- nitropropane
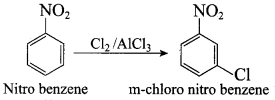
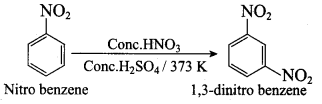
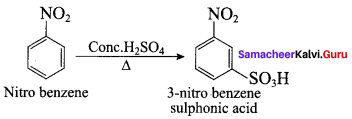
2, 2 – dimethyl – 1- nitropropane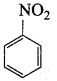 Nitrobenzene
Nitrobenzene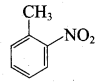 2 – nitro – 1 – methyl benzene
2 – nitro – 1 – methyl benzene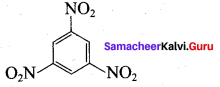 1, 3, 5 – trinitrobenzene
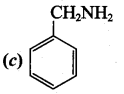
1, 3, 5 – trinitrobenzene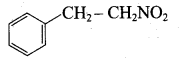 2 – phenyl – 1 – nitroethane
2 – phenyl – 1 – nitroethane
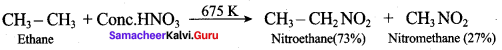
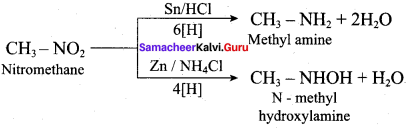
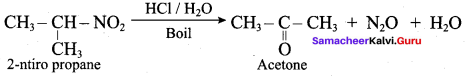
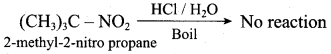
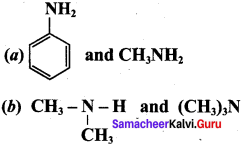
 Propan – 2 – amine
Propan – 2 – amine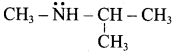
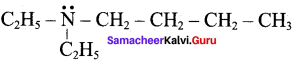
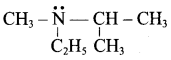
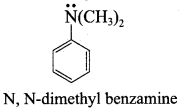
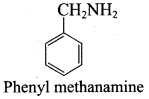
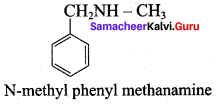
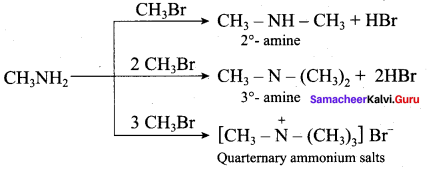
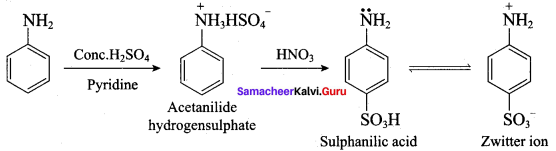
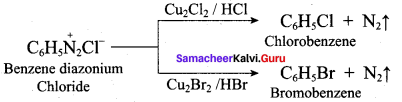
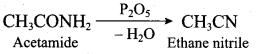
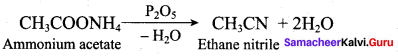
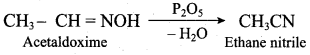
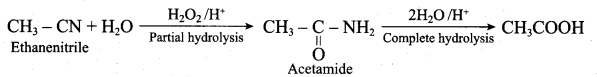
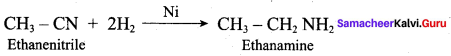
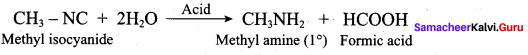
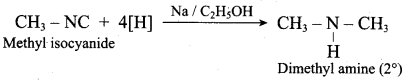
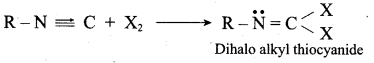
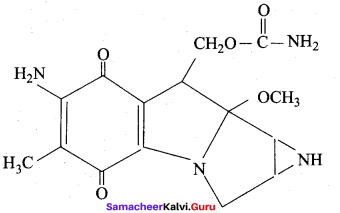
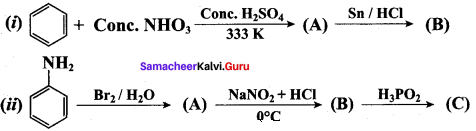
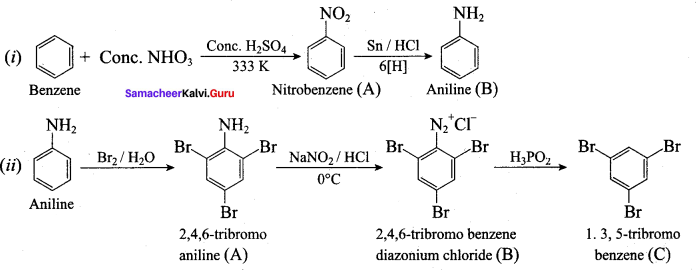
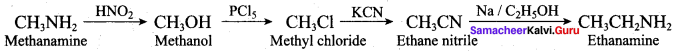
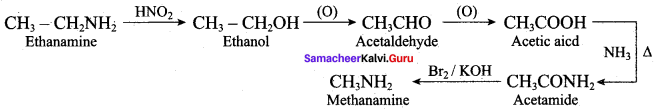
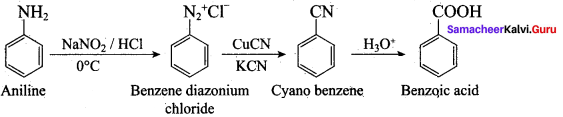
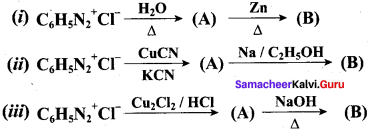
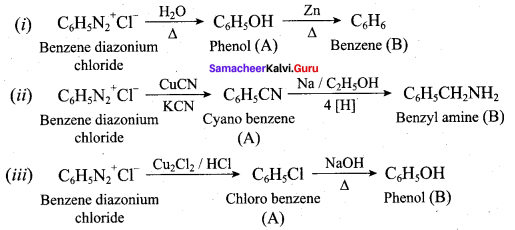

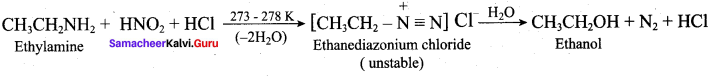
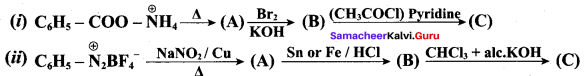
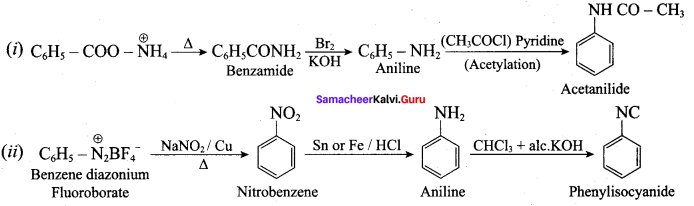
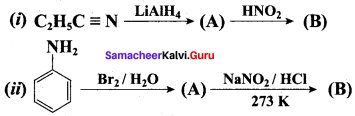
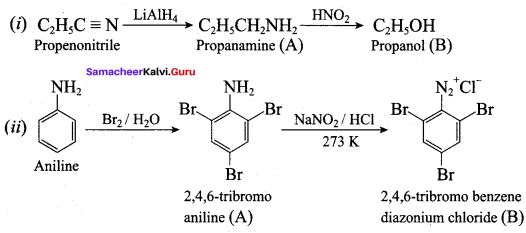
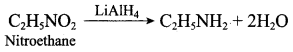
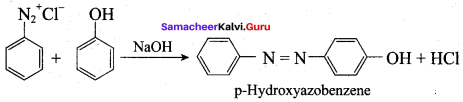
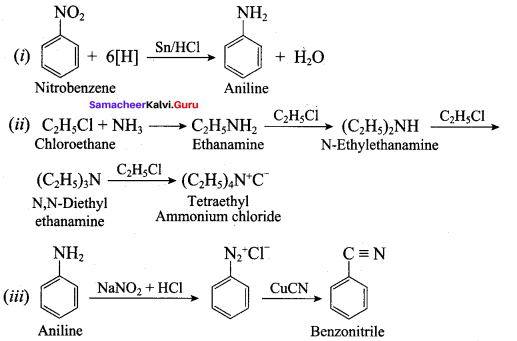
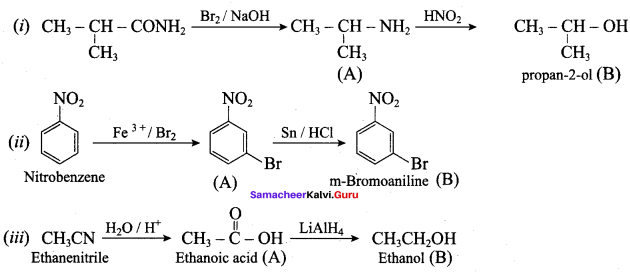
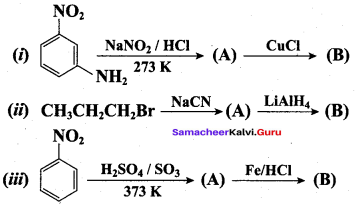
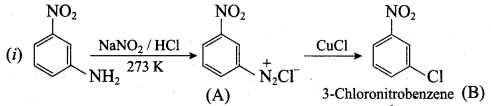
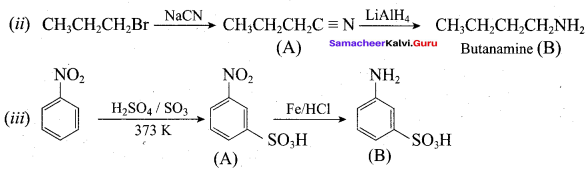
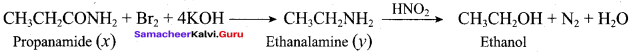
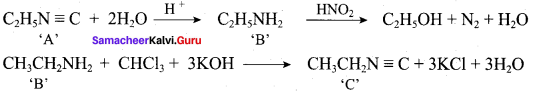
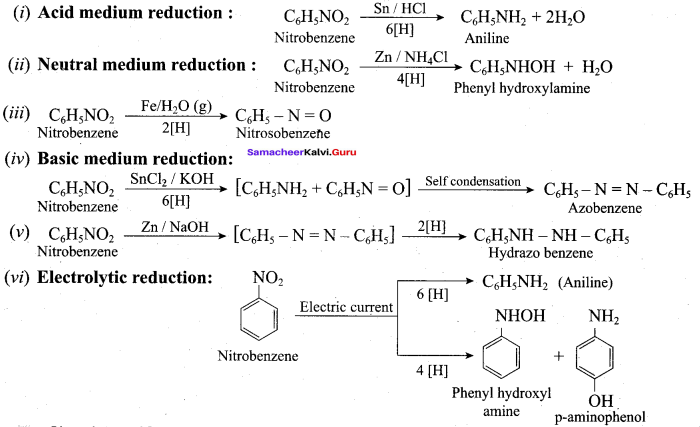
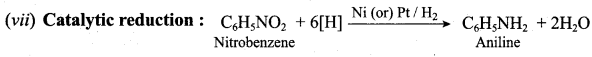
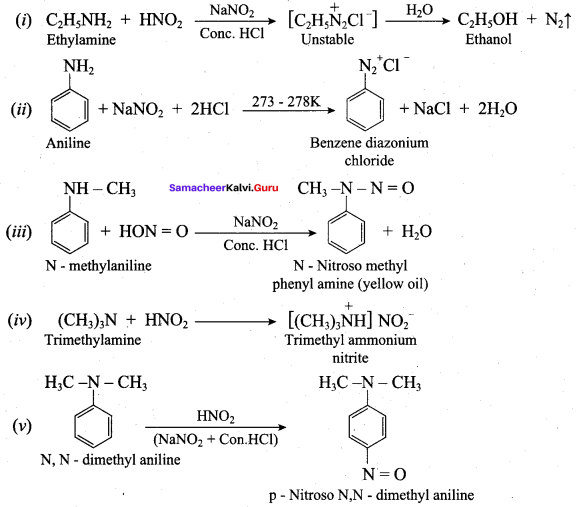
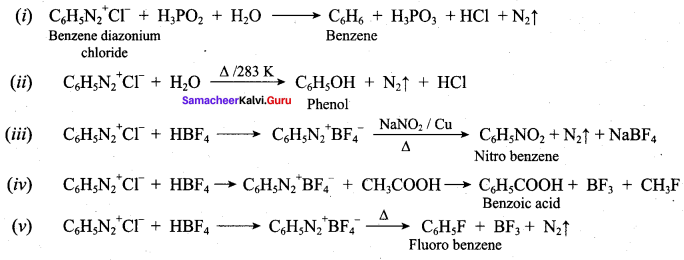
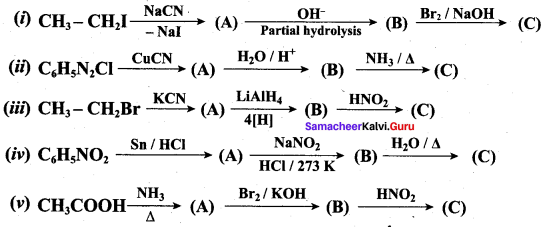
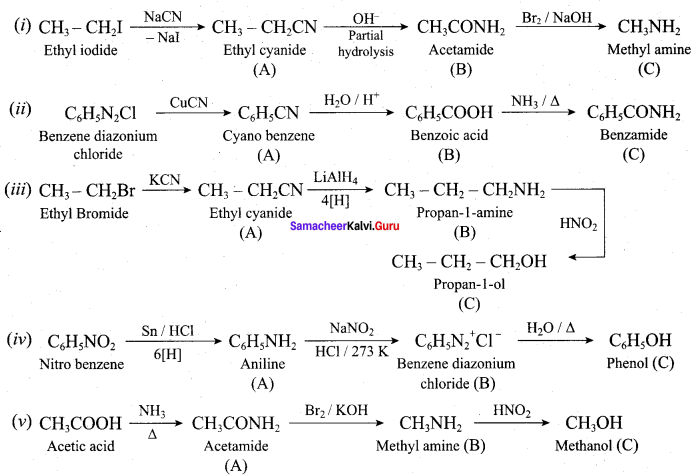

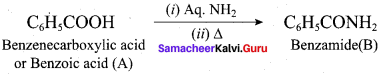
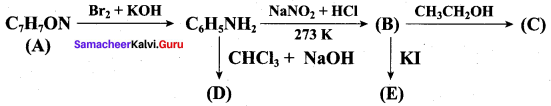
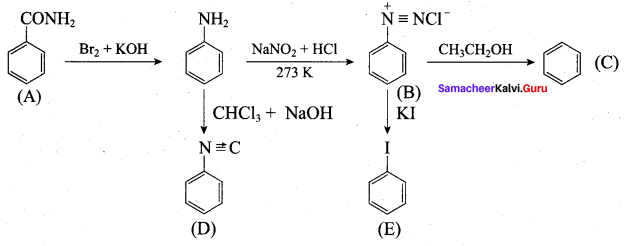
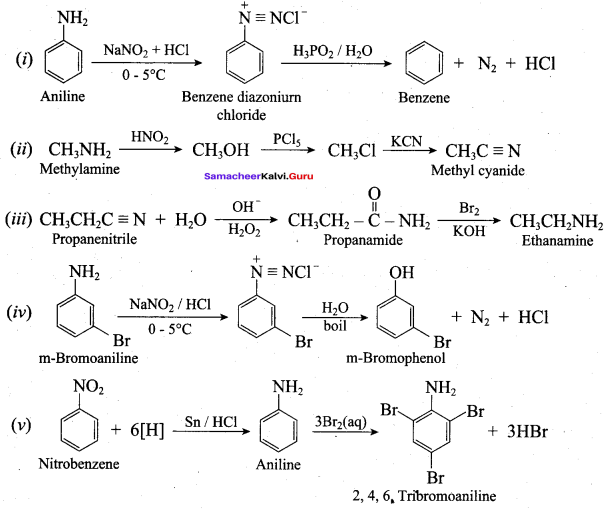
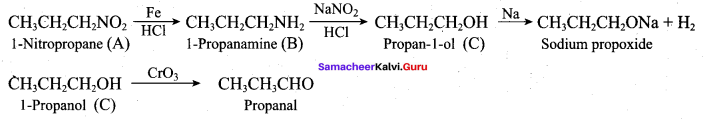
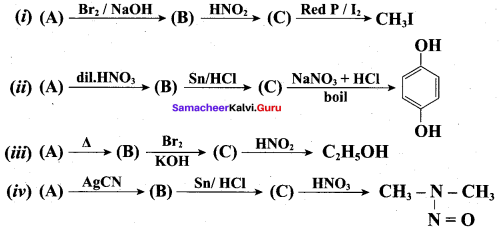
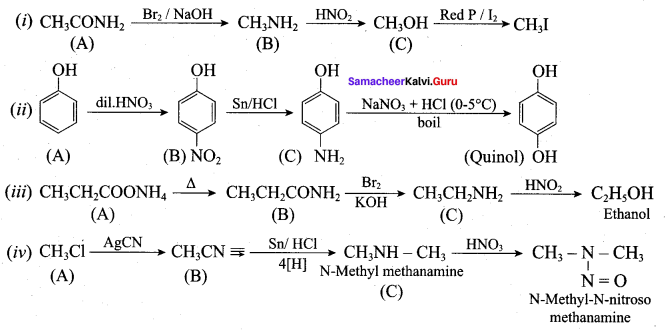
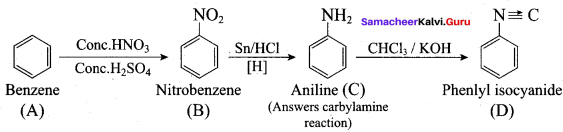
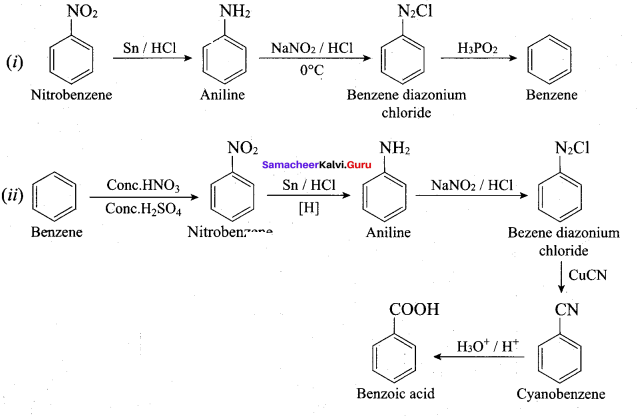


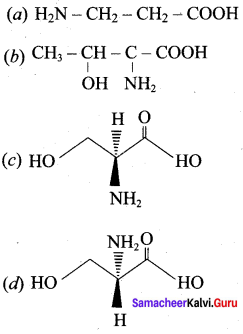
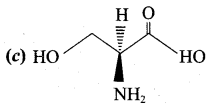
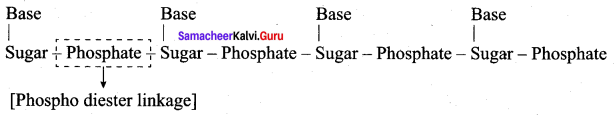
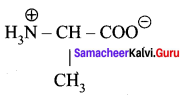
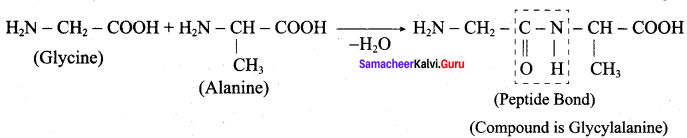
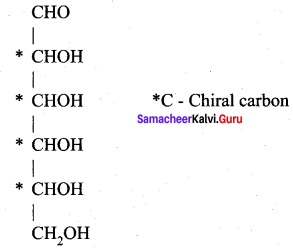
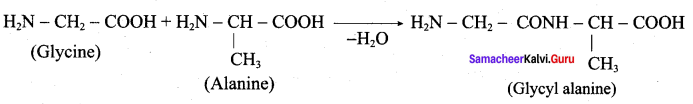
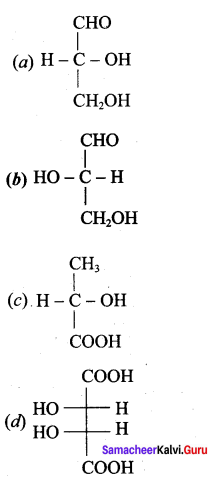
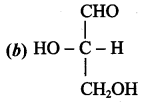
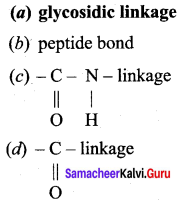
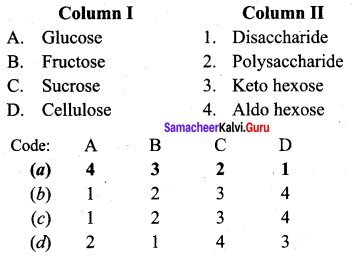




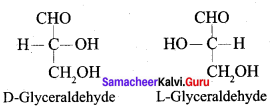
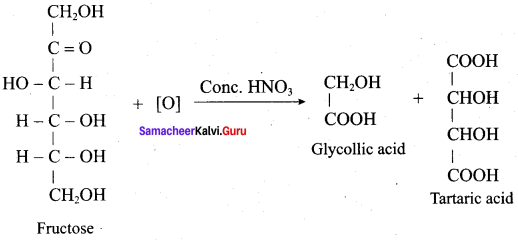
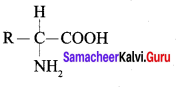
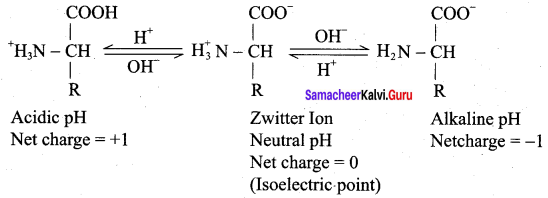
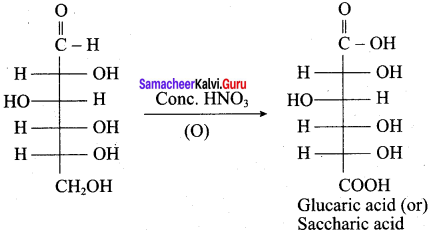
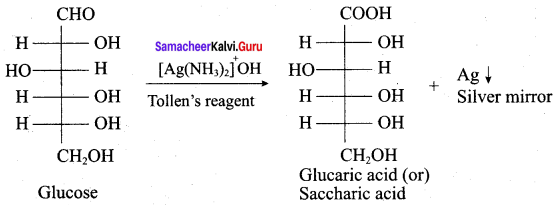
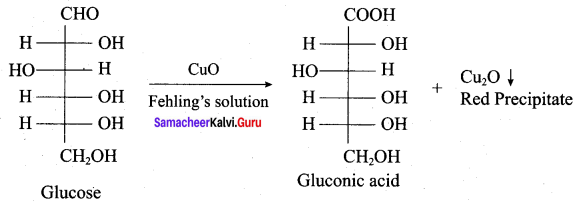
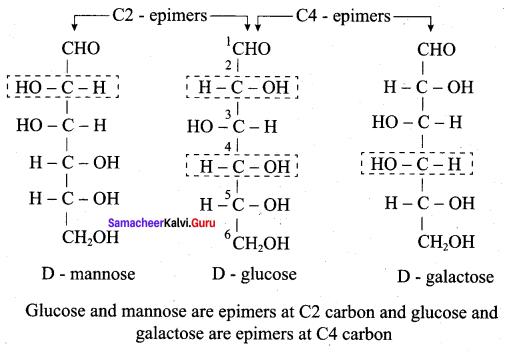
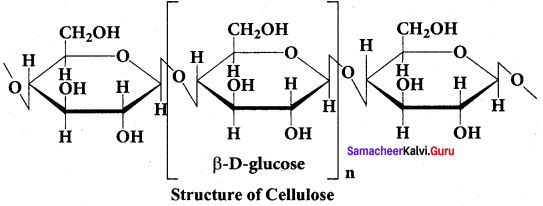

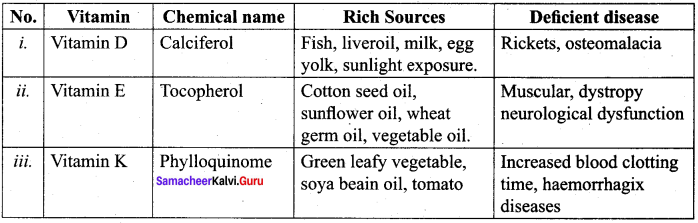
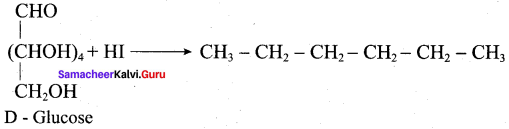
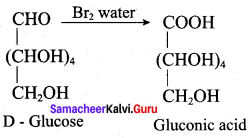



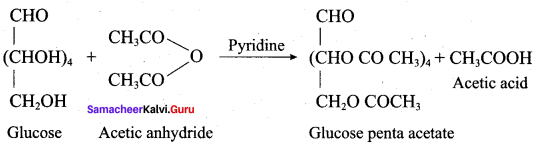
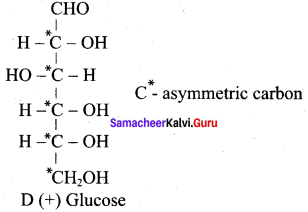
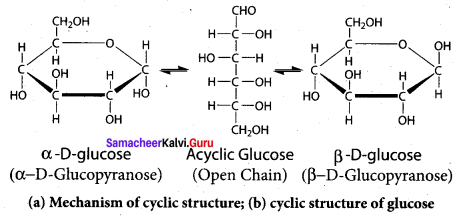

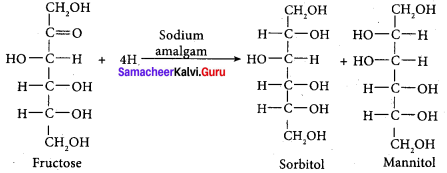



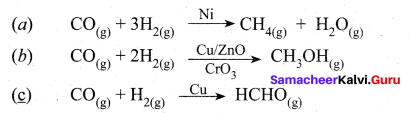
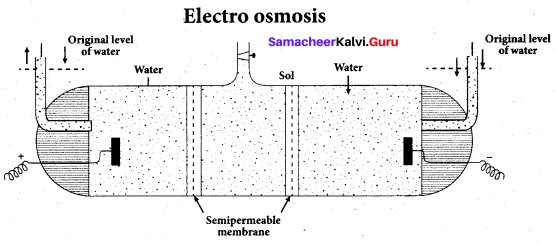

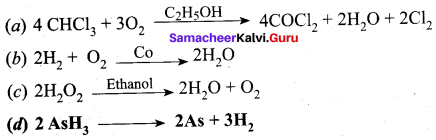
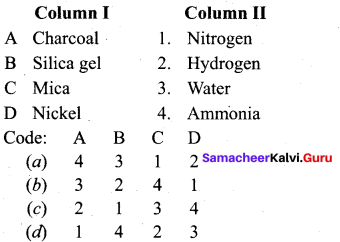
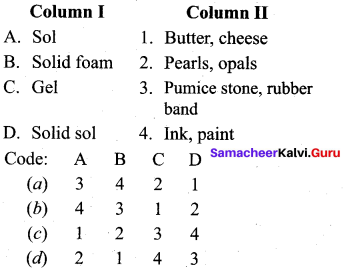
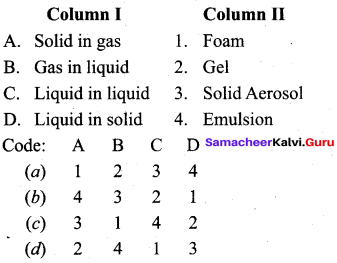
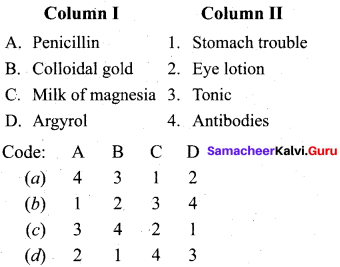
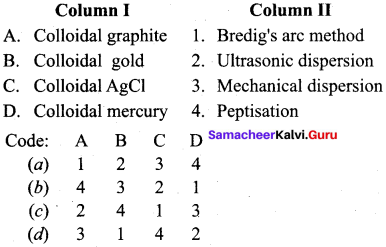




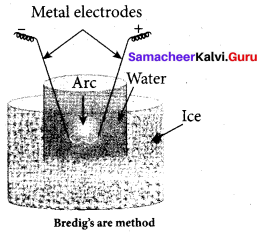
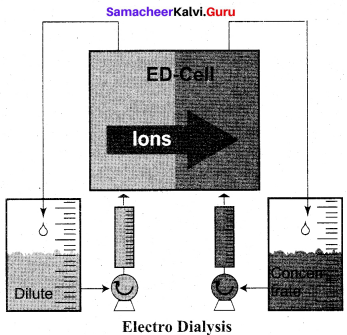
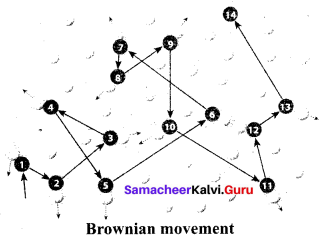
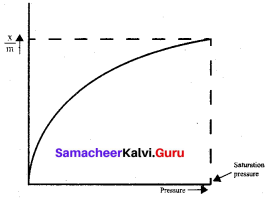

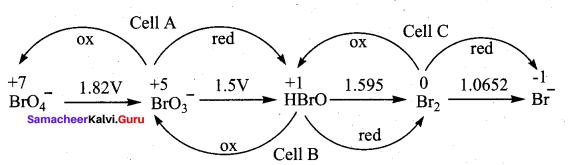
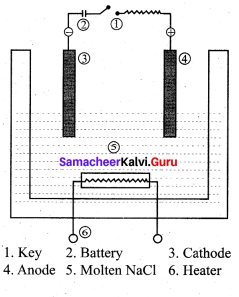
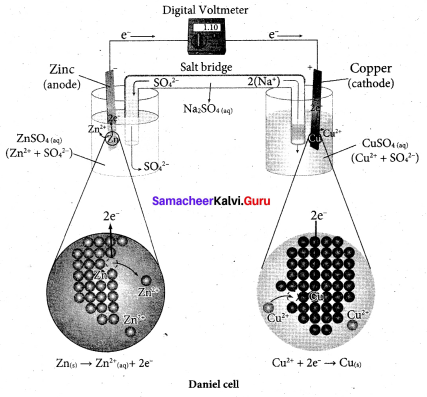
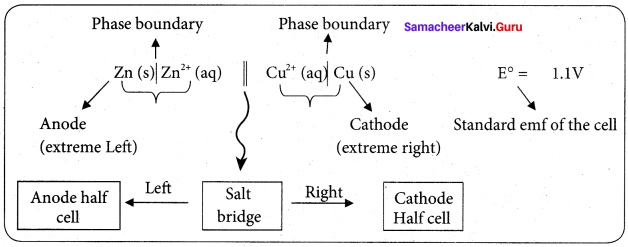

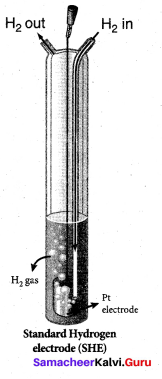
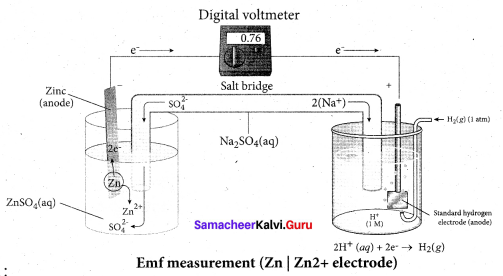
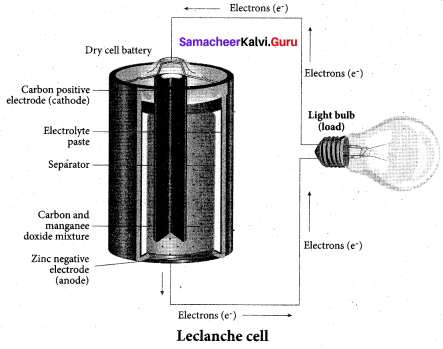
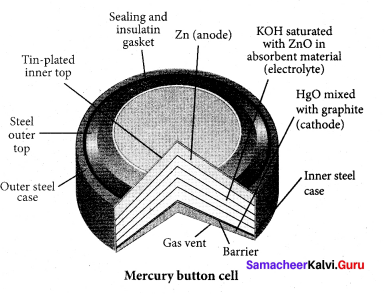
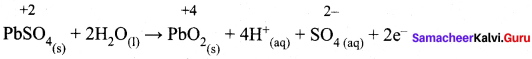
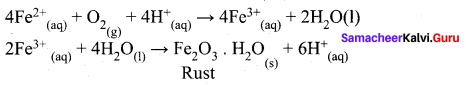
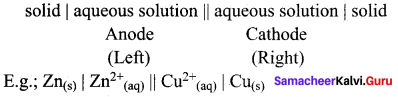
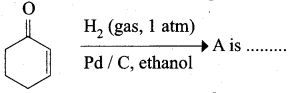
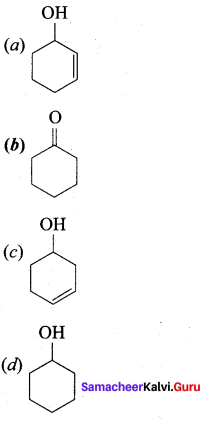
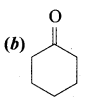
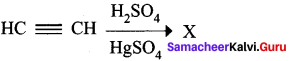
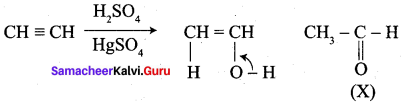
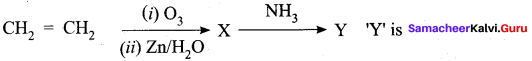
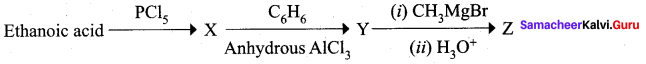
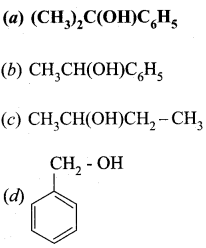
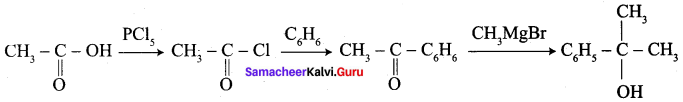
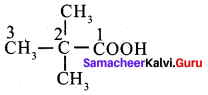
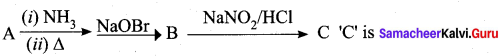
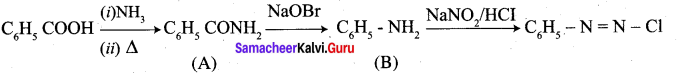
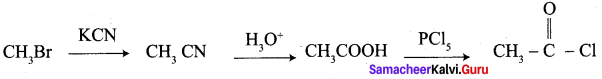

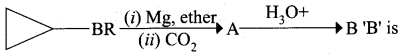
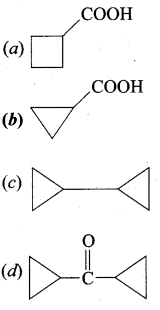
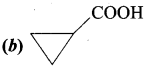
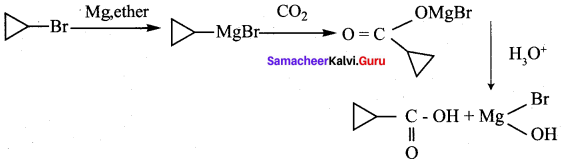
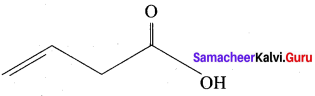 ……………………
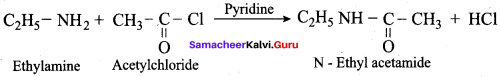
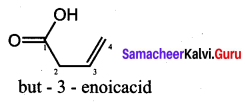
……………………
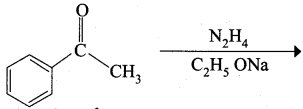
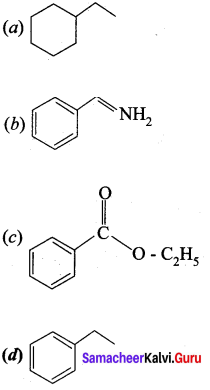
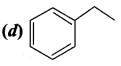
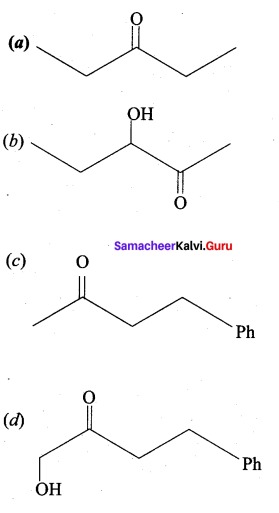
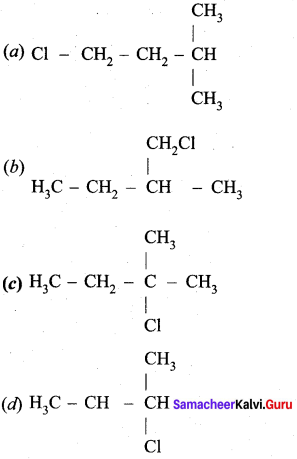
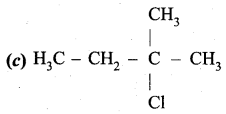
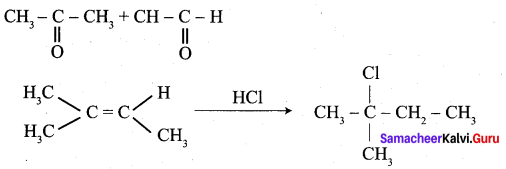
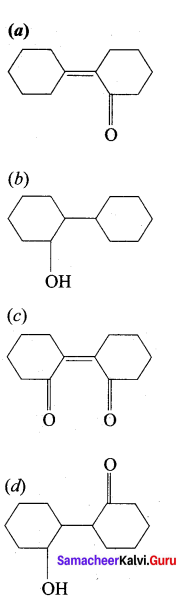
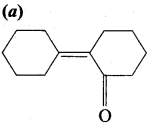
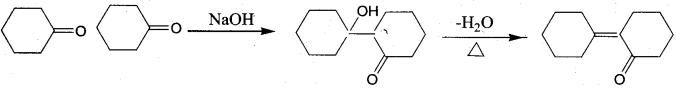
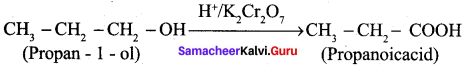
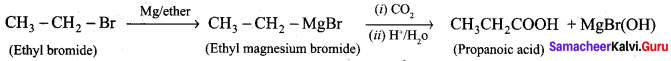

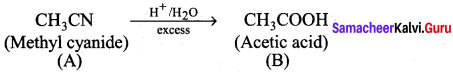


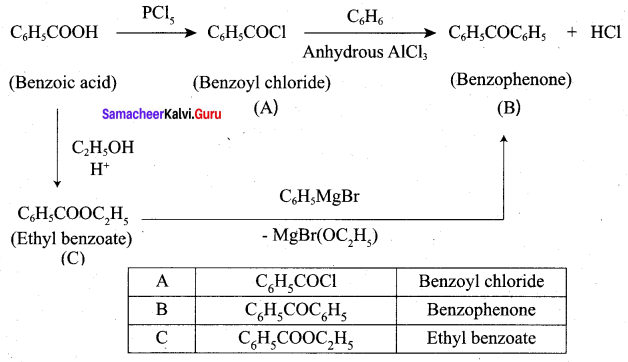
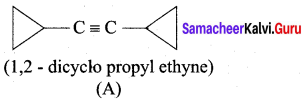
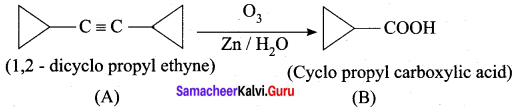
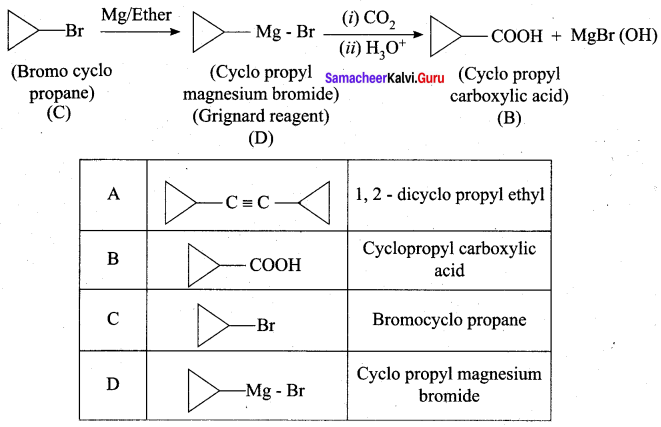
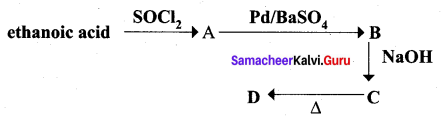
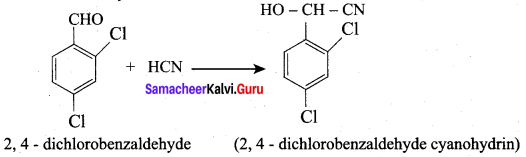
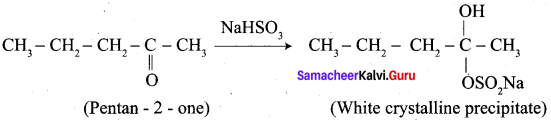
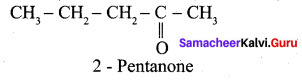
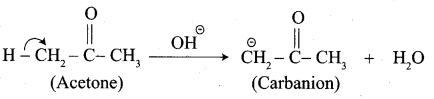
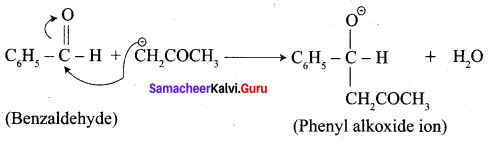
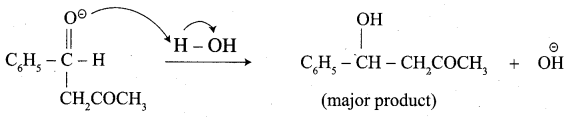
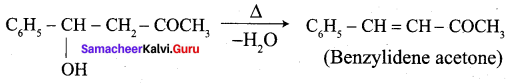
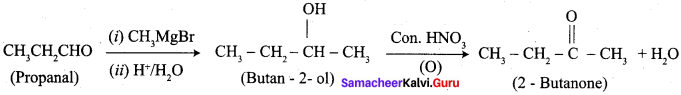
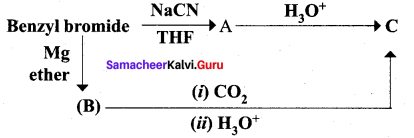
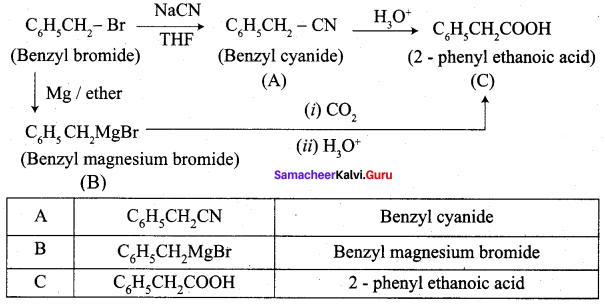
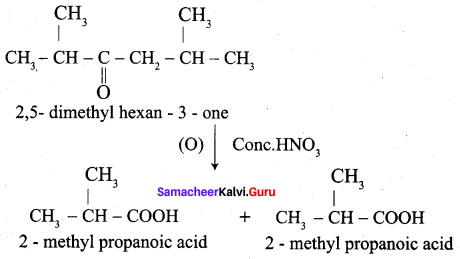
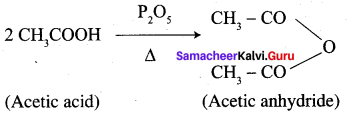
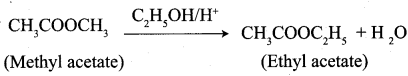
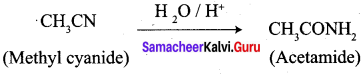

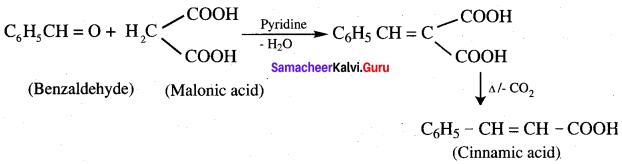
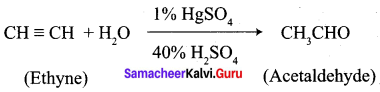
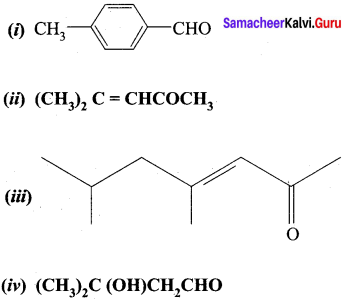
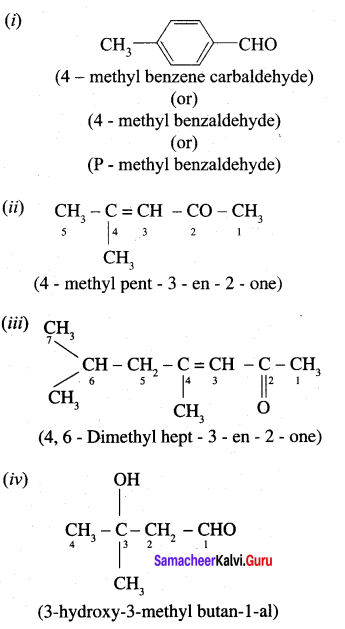
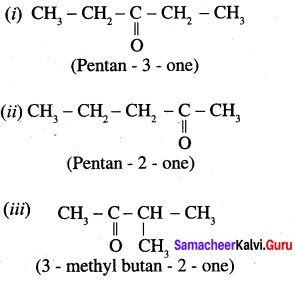
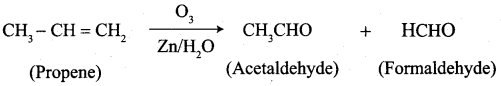
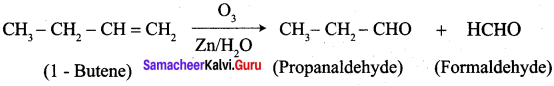
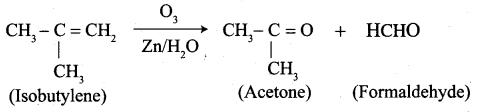
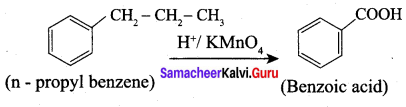
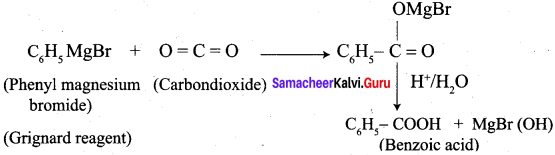
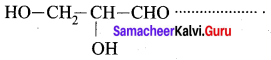 …………………..
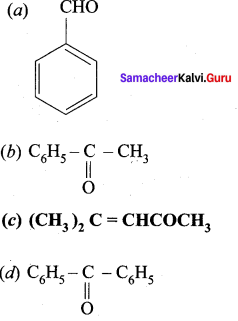
…………………..
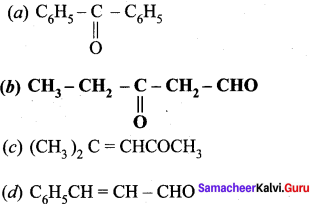
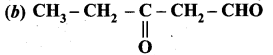
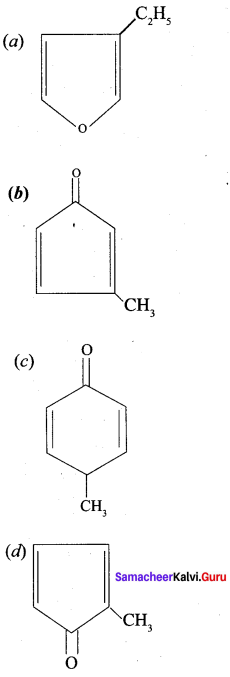
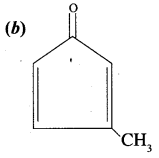
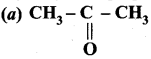
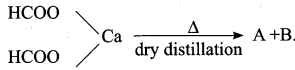
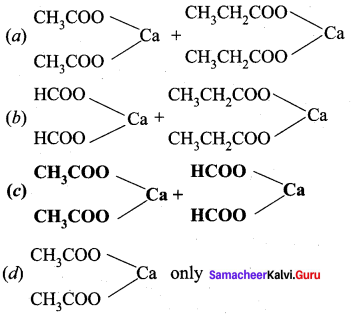
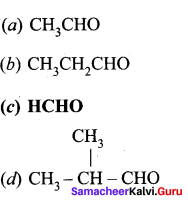
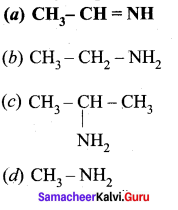
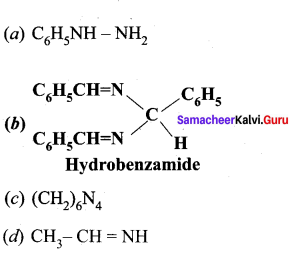
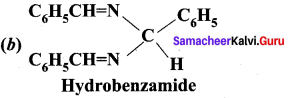
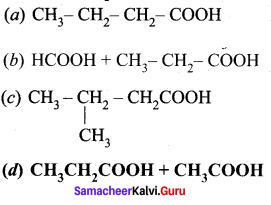
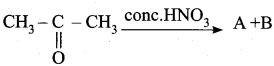
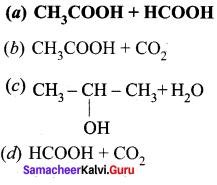
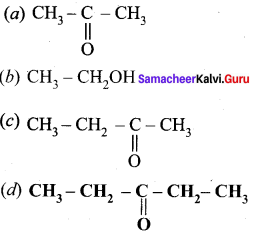
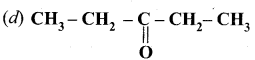
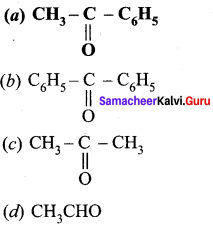
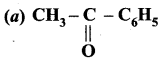

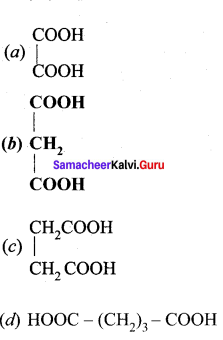
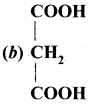
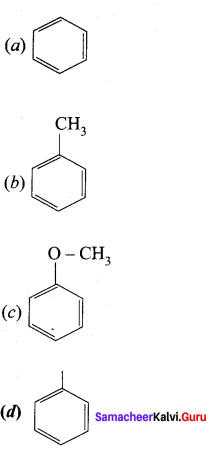
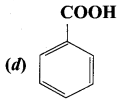
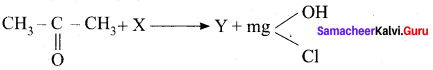
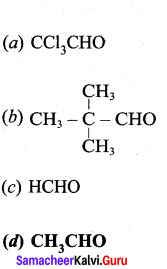
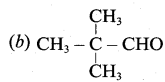
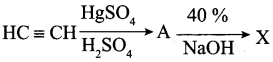
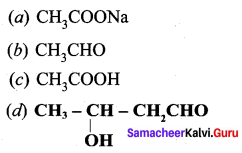
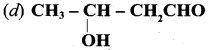
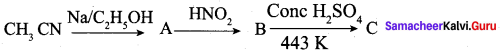
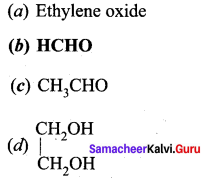
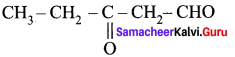 is ………….
is ………….
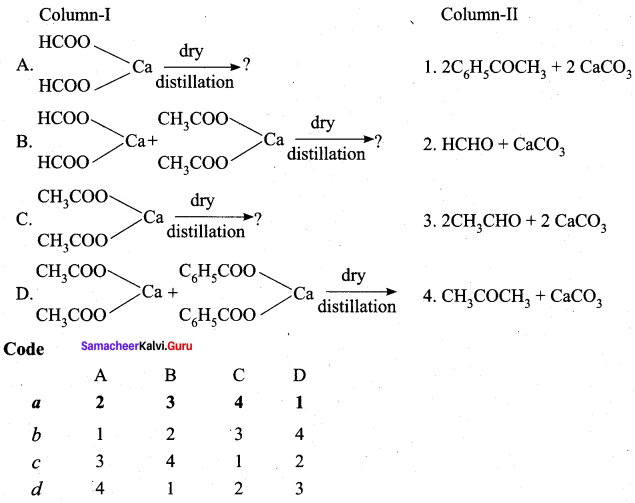
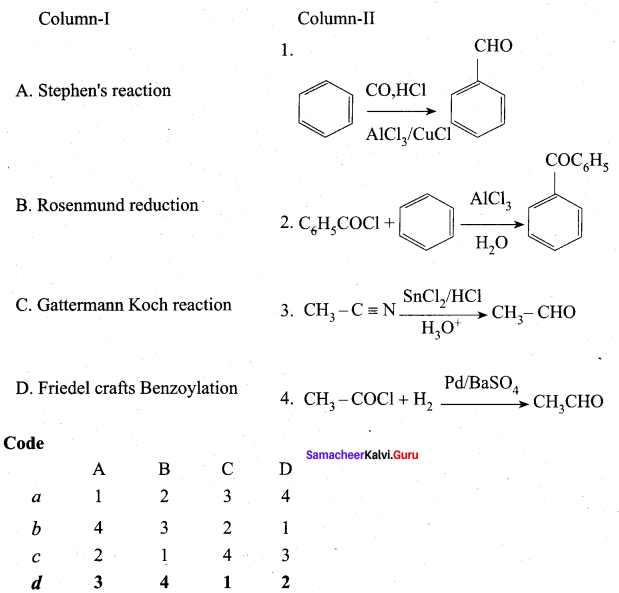
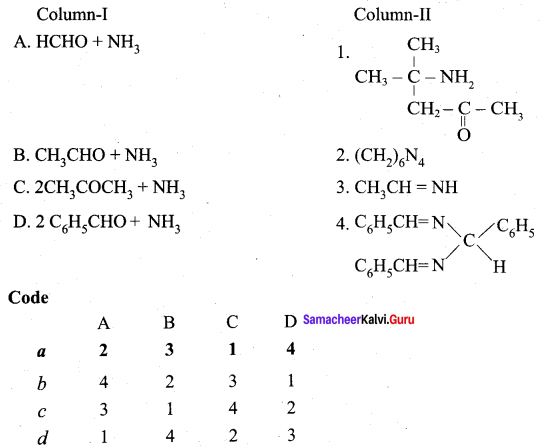
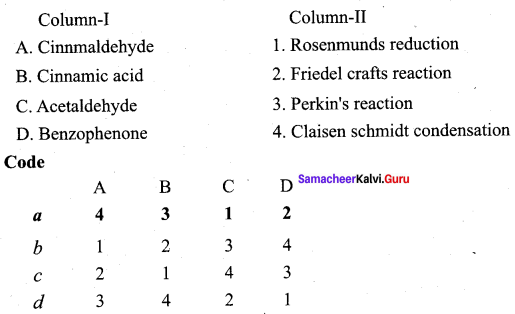
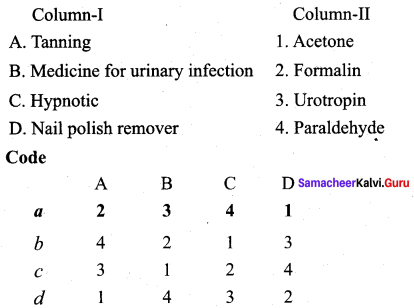
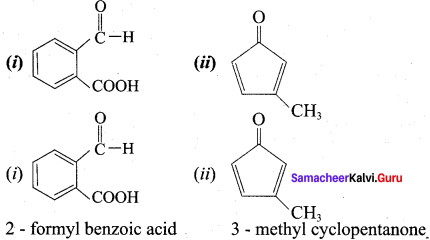
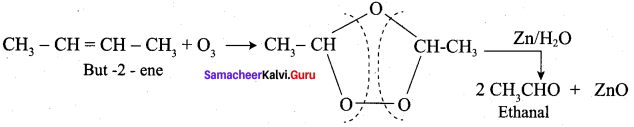
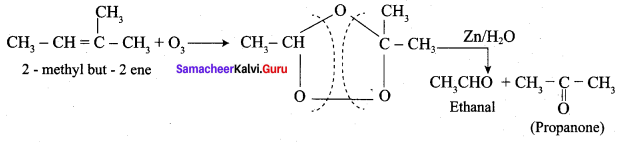
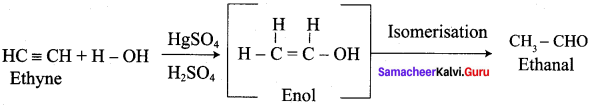
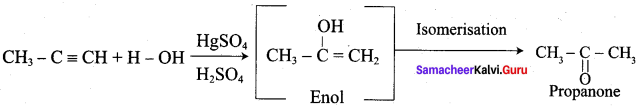
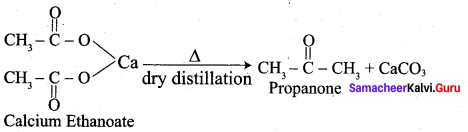
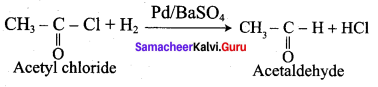
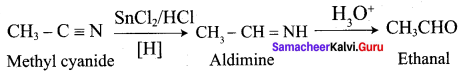

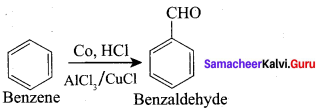
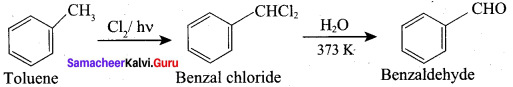
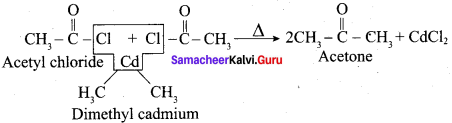
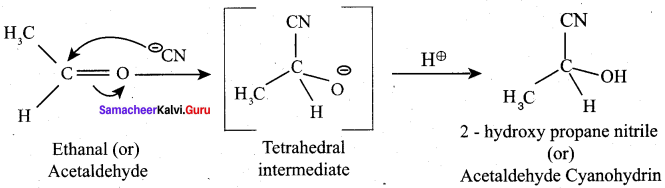
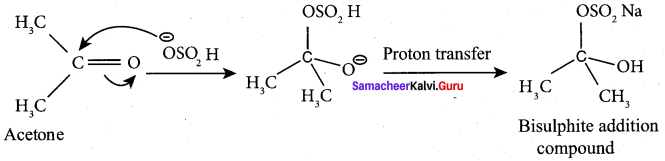
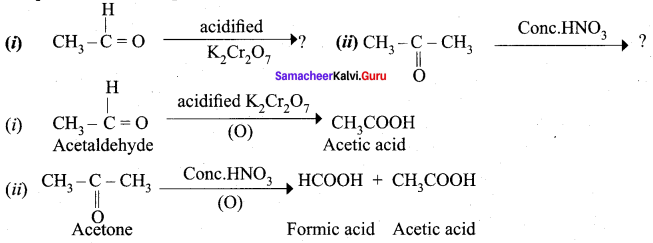
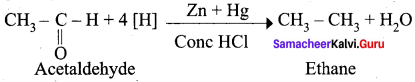
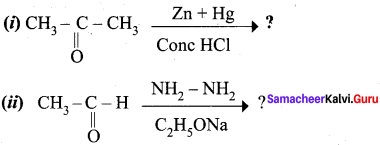
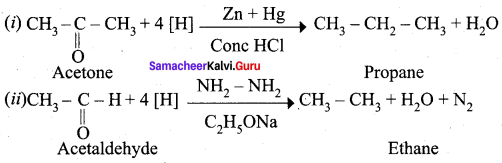
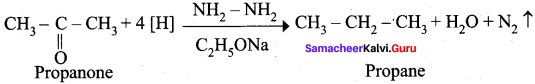
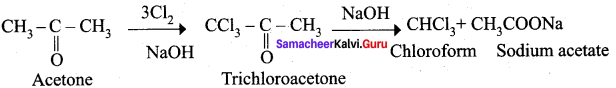
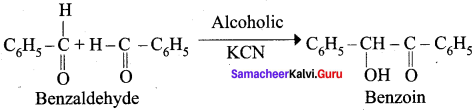
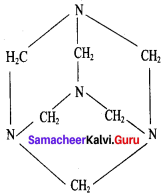
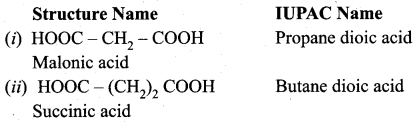
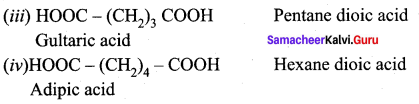
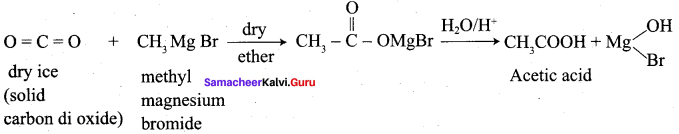
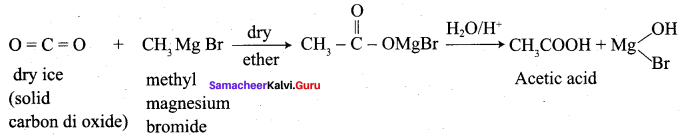
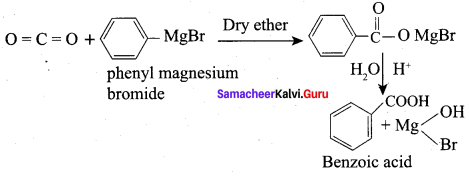
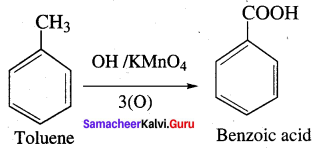
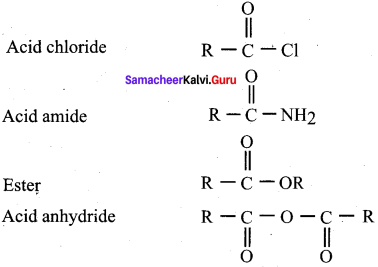
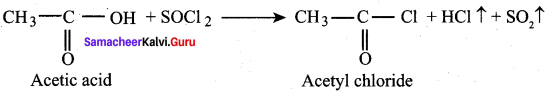

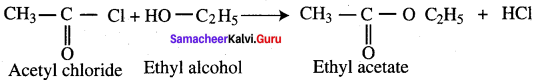
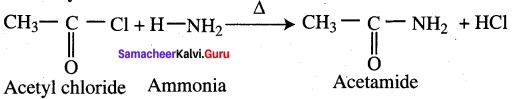
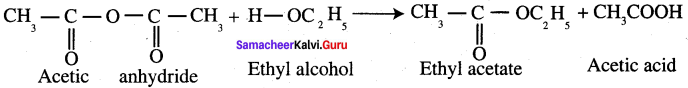

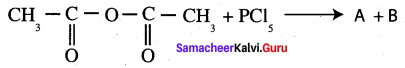
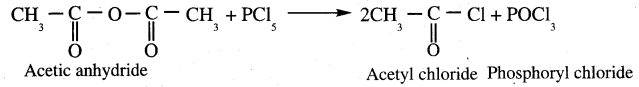
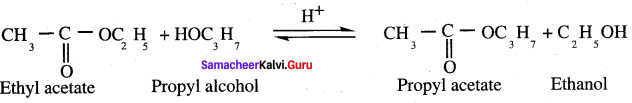

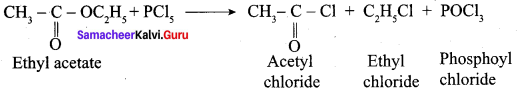
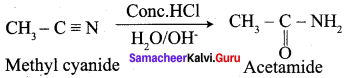
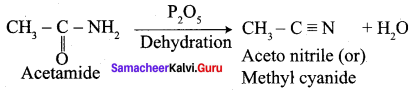
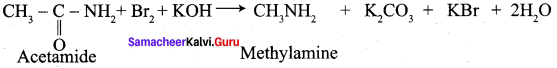
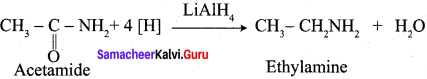
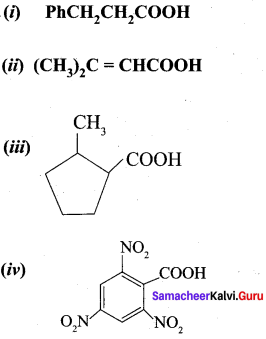
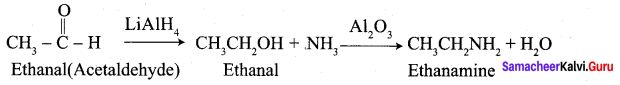
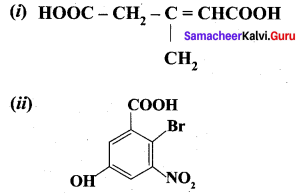
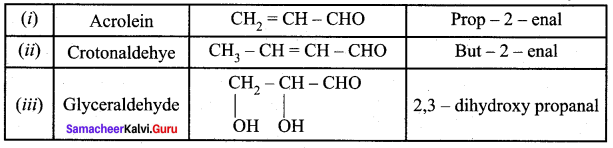
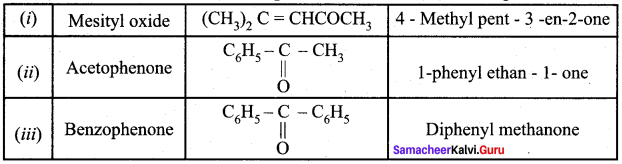
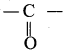
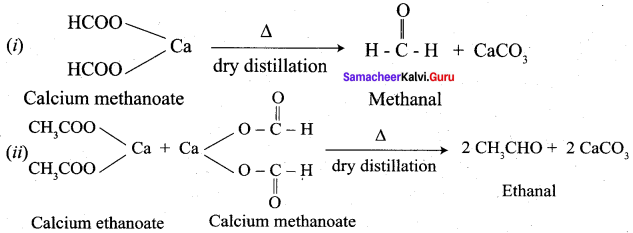
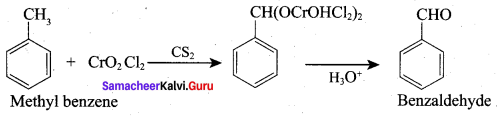
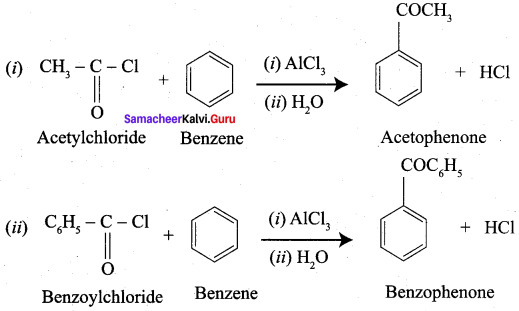
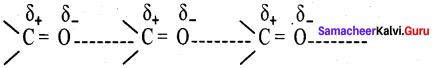
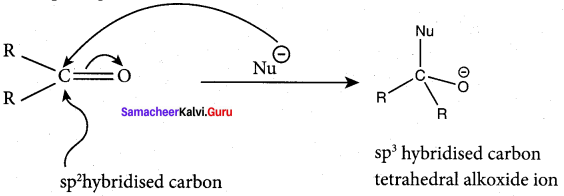
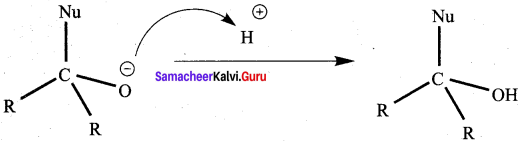
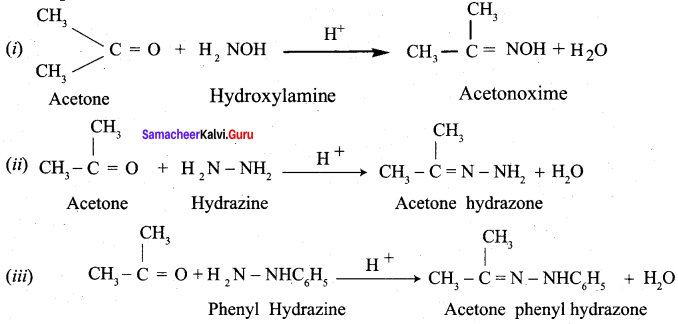
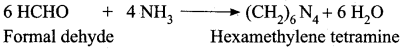
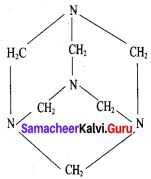
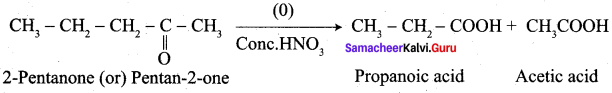
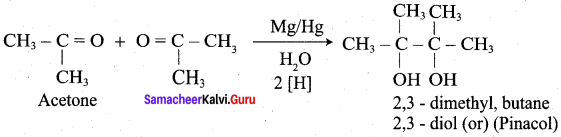
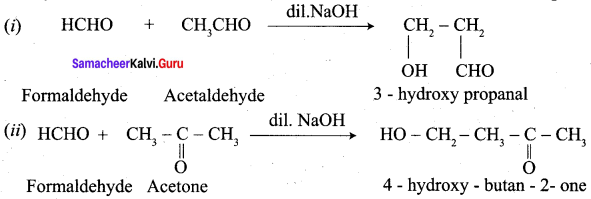
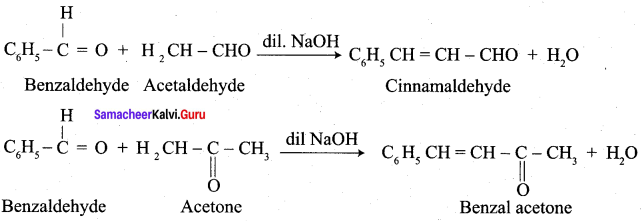

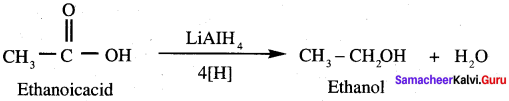
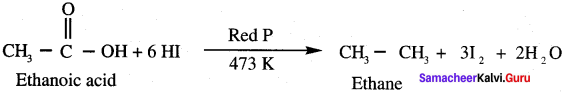
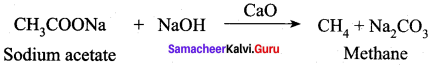
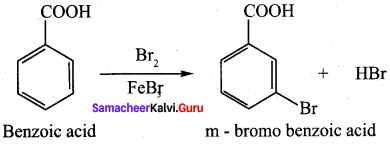
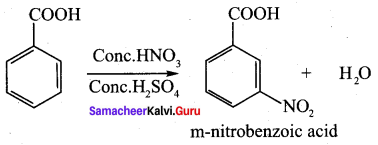
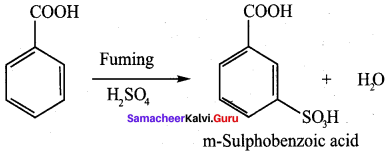
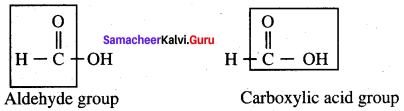




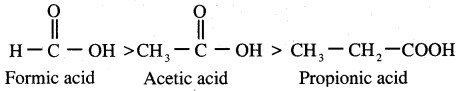
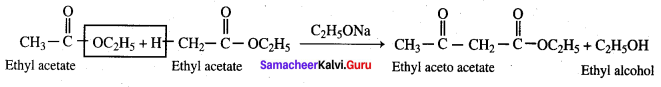
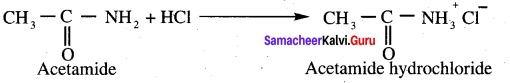
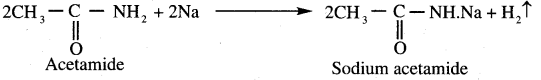
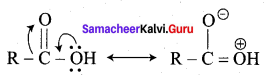
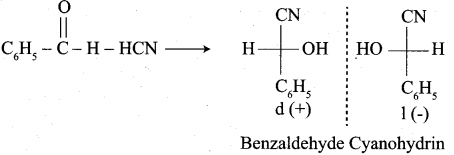
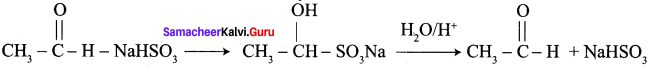
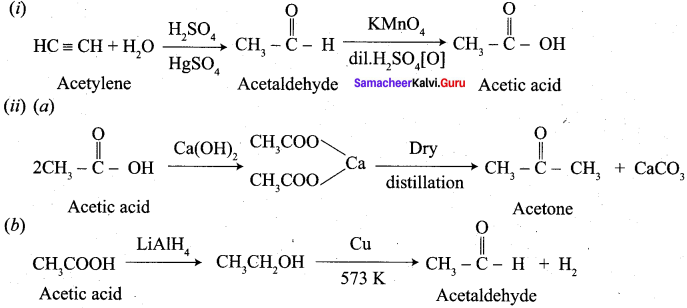
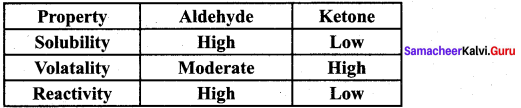
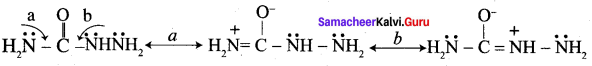
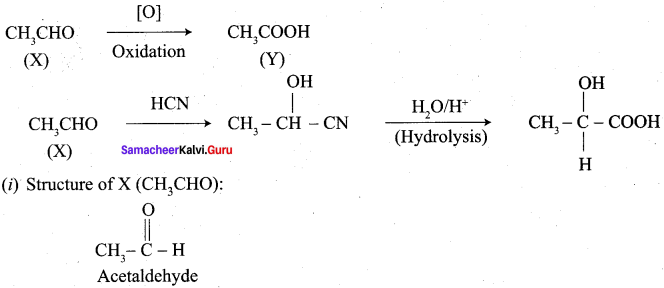
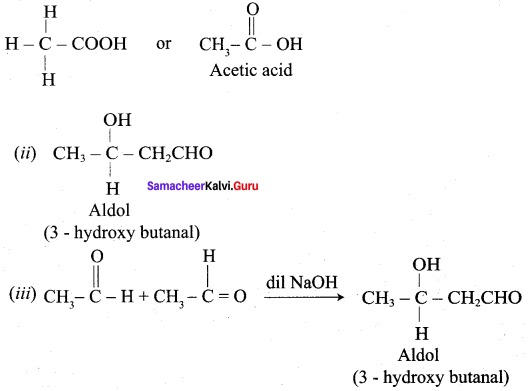
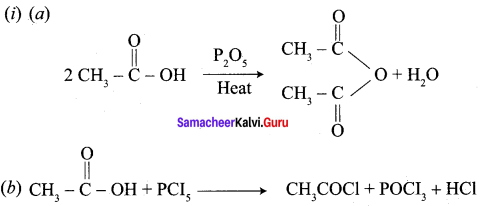
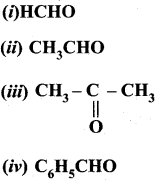
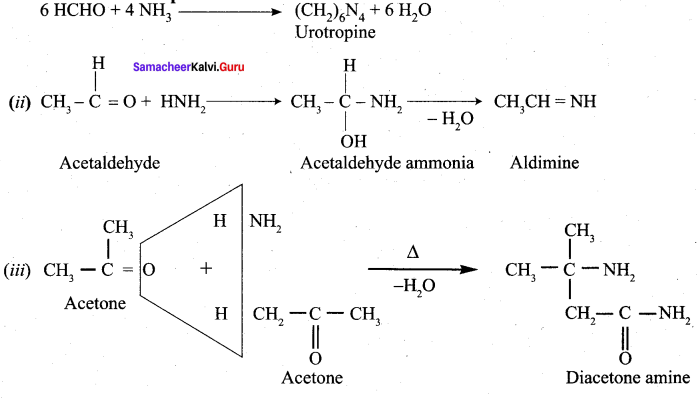
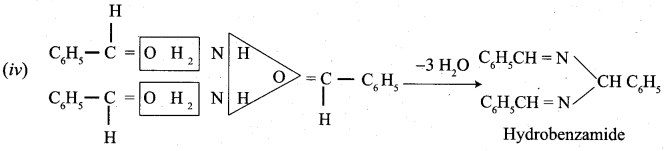
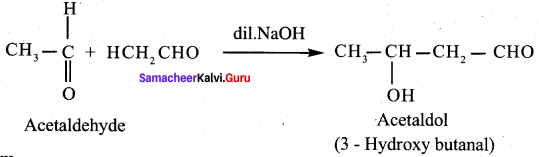

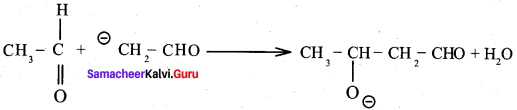
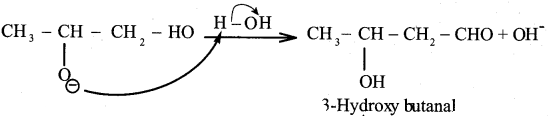
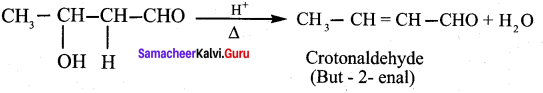
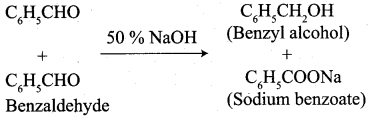
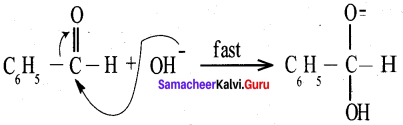
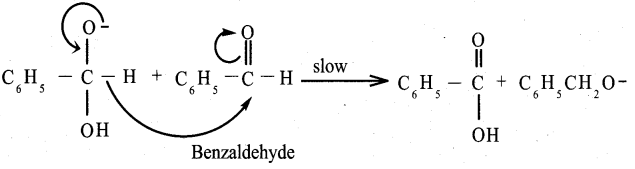
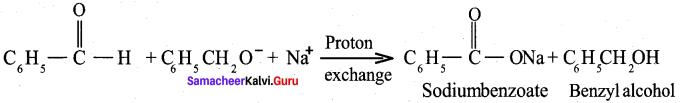
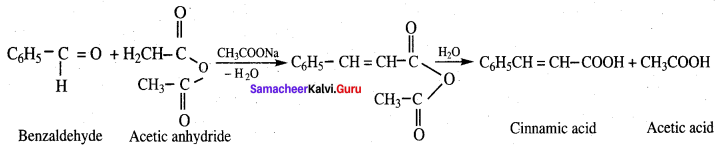
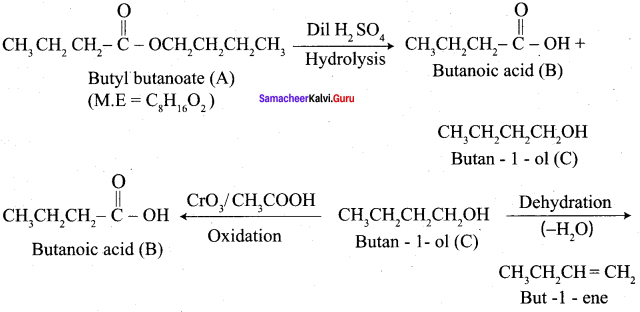
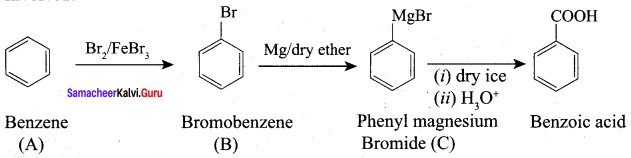
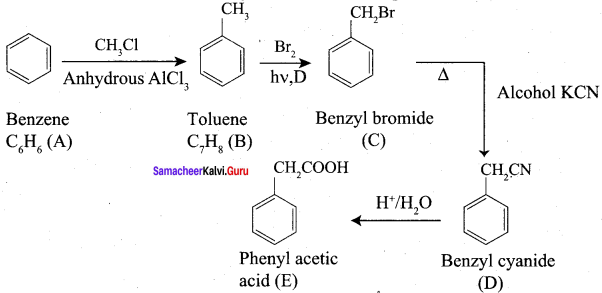
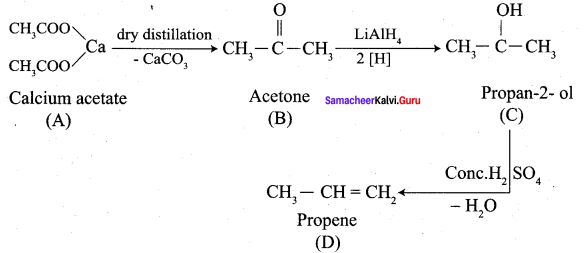
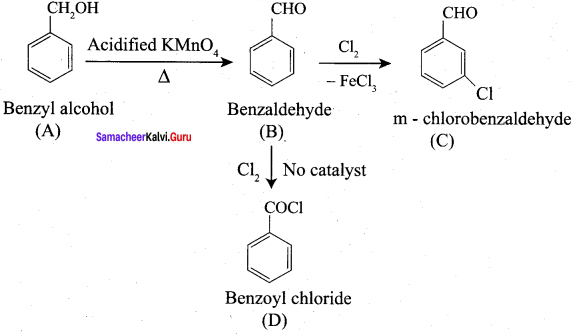
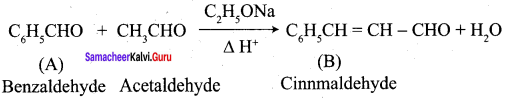
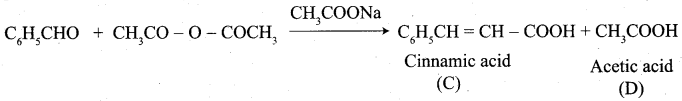
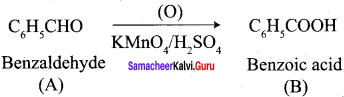
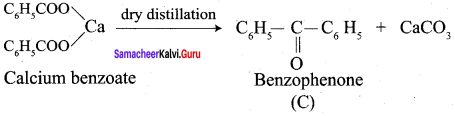
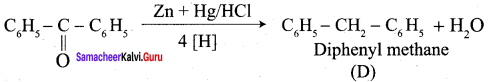
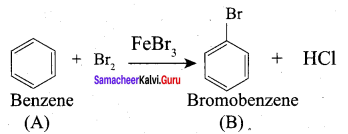
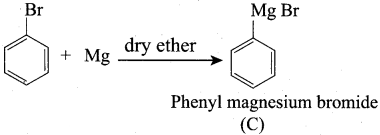
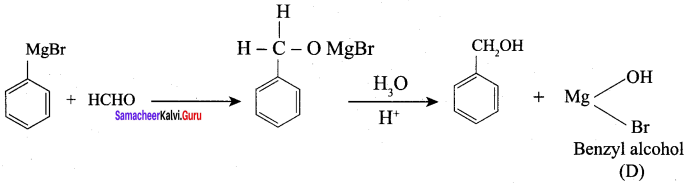
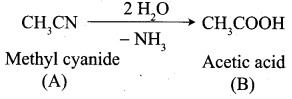
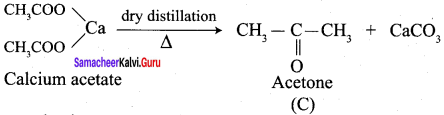
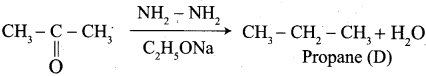
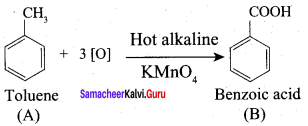
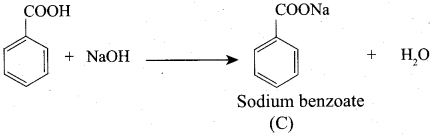
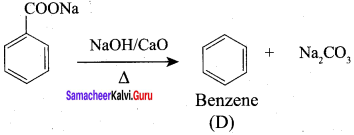
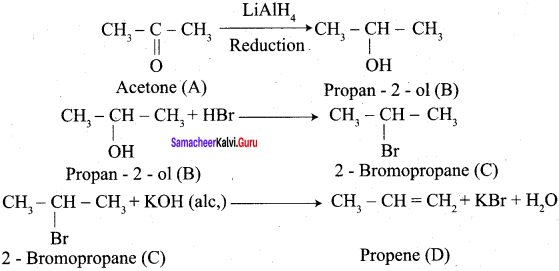
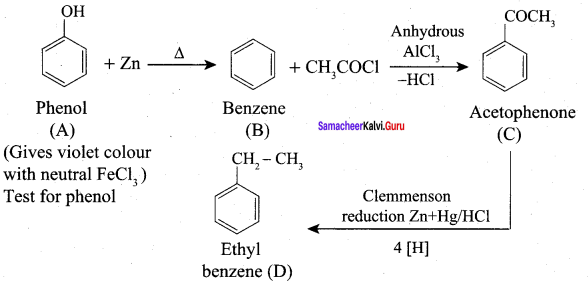
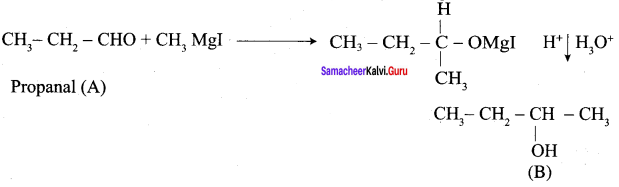
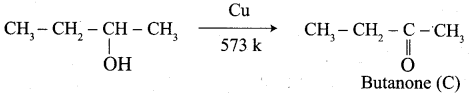
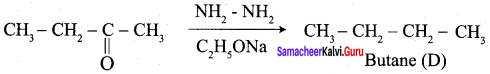
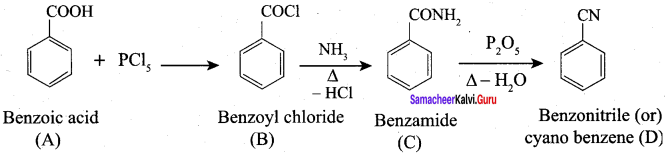
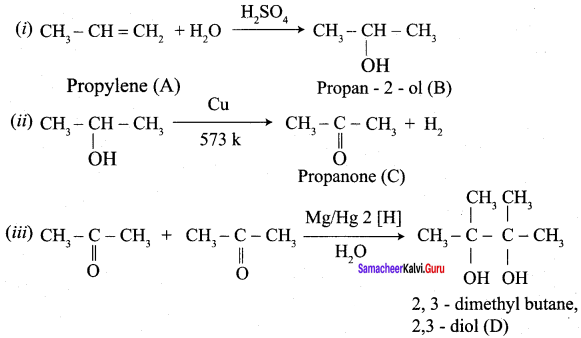
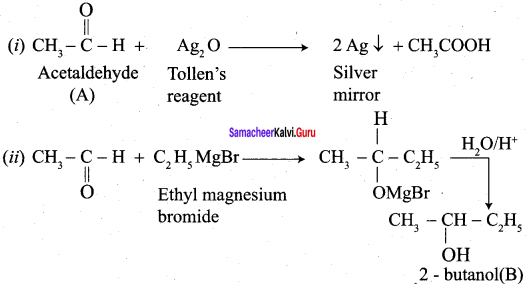
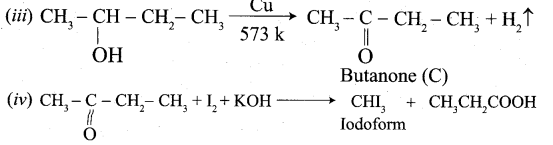
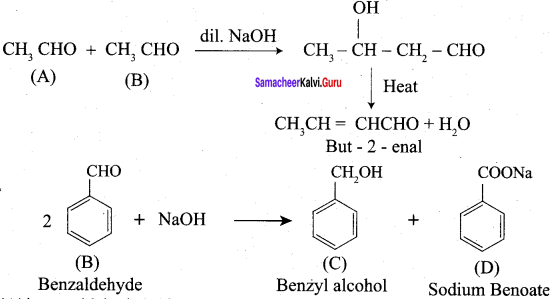
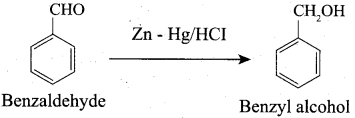
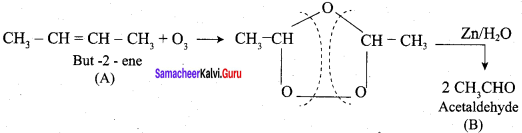
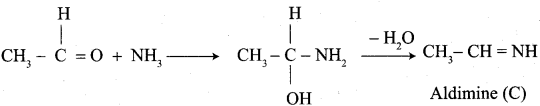
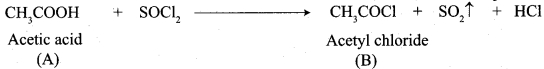
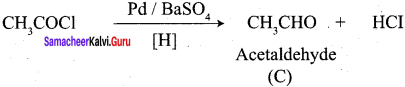
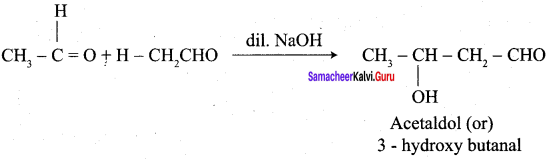
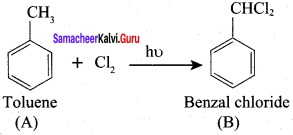
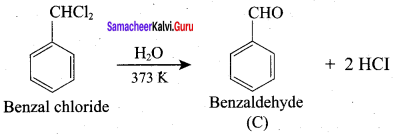
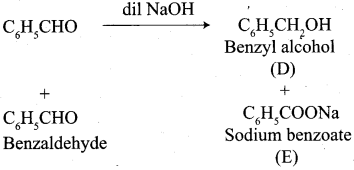
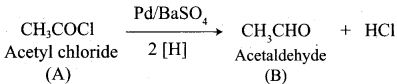
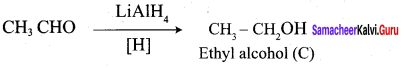
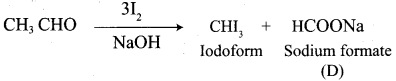
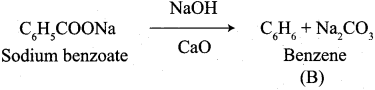
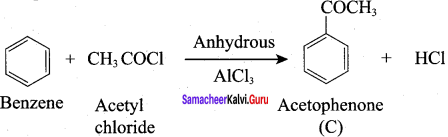
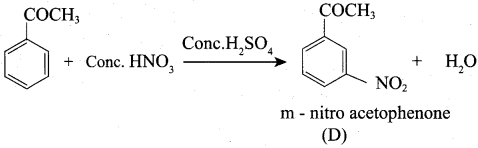


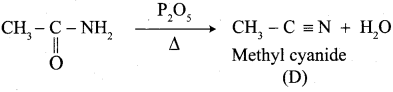
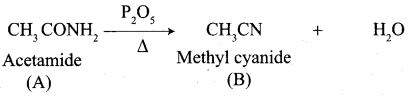
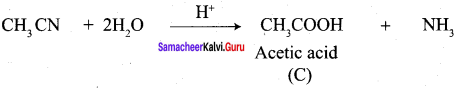
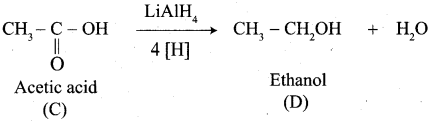
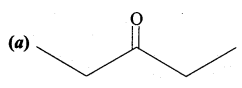
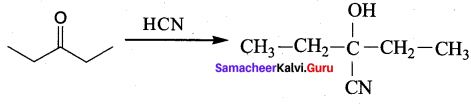
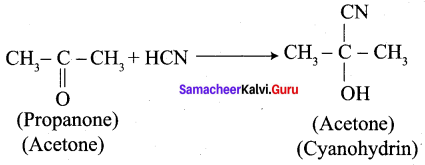
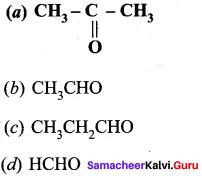
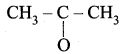 Propanone
Propanone