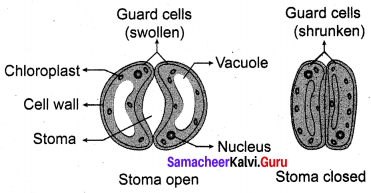
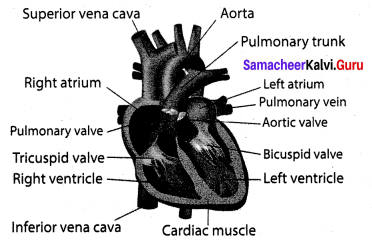
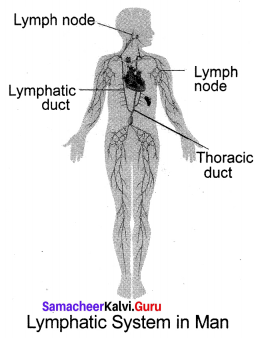
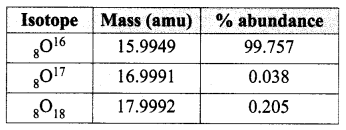
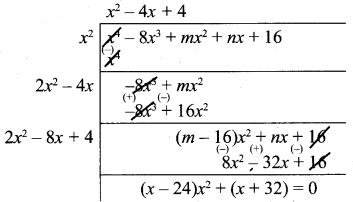You can Download Samacheer Kalvi 10th Maths Book Solutions Guide Pdf, Tamilnadu State Board help you to revise the complete Syllabus and score more marks in your examinations.
Tamilnadu Samacheer Kalvi 10th Maths Solutions Chapter 3 Algebra Ex 3.2
10th Maths Exercise 3.2 Samacheer Kalvi Question 1.
Find the GCD of the given polynomials
(i) x4 + 3x3 – x – 3, x3 + x2 – 5x + 3
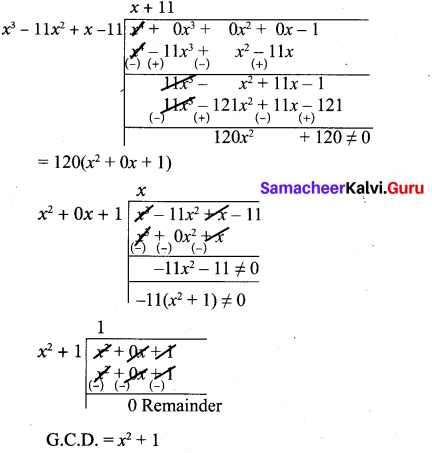
(ii) x4 – 1, x3 – 11x2 + x – 11
(iii) 3x4 + 6x3 – 12x4 – 24x, 4x4 + 14x3 + 8x2 – 8x
(iv) 3x3 + 3x2 + 3x + 3, 6x3 + 12x2 + 6x + 12
Solution:
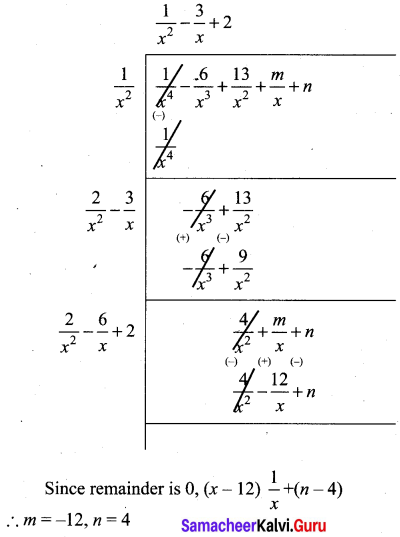
x4 + 3x3 – x – 3, x3 + x2 – 5x + 3
Let f(x) = x4 + 3x3 – x – 3
g(x) = x3 + x2 – 5x + 3
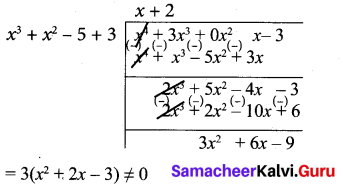
Note that 3 is not a divisor of g(x). Now dividing g(x) = x3 + x2 – 5x + 3 by the new remainder x2 + 2x – 3 (leaving the constant factor 3) we get
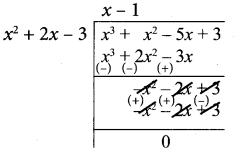
Here we get zero remainder
G.C.D of (x4 + 3x3 – x – 3), (x3 + x2 – 5x + 3) is (x2 + 2x – 3)
(ii) x4 – 1, x3 – 11x2 + x – 11
(iii) 3x4 + 6x3 – 12x2 – 24x, 4x4 + 14x3 + 8x2 – 8x
4x4 + 14x3 + 8x2 – 8x = 2 (2x4 + 7x3 + 4x2 -4x)
Let us divide
(2x4 + 7x3 + 4x2 + 4x) by x4 + 2x3 – 4x2 – 8x

(x3 + 4x3 + 4x) ≠ 0
Now let us divide
x4 + 2x3 – 4x2 – 8x by x3 + 4x2 + 4x
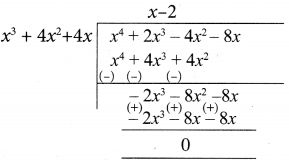
∴ x3 + 4x2 + 4x is the G.C.D of 3x4 + 6x3 -12x2 – 24x, 4x4 + 14x3 + 8x2 -8x
∴ Ans x (x2 + 4x + 4)
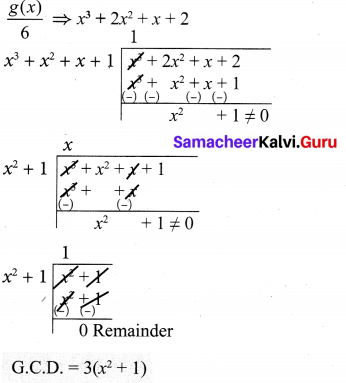
(iv) f(x) = 3x3 + 3x2 + 3x + 3 = 3(x3 + x2 + x + 1)
g(x) = 6x3 + 12x2 + 6x + 12
= 6(x3 + 2x2 + x + 2)
= 2 × 3 (x3 + 2x2 + x + 2)
f(x) ⇒ x3 + x2 + x + 1
Ex 3.2 Class 10 Samacheer Question 2.
Find the LCM of the given expressions,
(i) 4x2y, 8x3y2
(ii) -9a3b2, 12a2b2c
(iii) 16m, -12m2n2, 8n2
(iv) p2 – 3p + 2, p2 – 4
(v) 2x2 – 5x – 3, 4x2 – 36
(vi) (2x2 – 3xy)2, (4x – 6y)3, 8x3 – 27y3
Solution:
(i) 4x2y, 8x3y2
4x2y = 2 × 2 x2y
8x3y2 = 2 × 2 × 2 x3y2
L.C.M. = 2 × 2 × 2 x3y2
= 8x3 y2
(ii) -9a3b2 = -3 × 3 a3b2
12a2b2c = 2 × 3 × 2a2b2c
L.C.M. = -3 × 3 × 2 × 2 a3b2c
= -36a3b2c
(iii) 16m, -12m2n2, 8n2
16 m = 2 × 2 × 2 × 2 × m
-12m2n2 = -2 × 2 × 3 × m2n2
8n2 = 2 × 2 × 2 × n2
L.C.M.= -2 × 2 × 2 × 2 × 3 m2n2
= -48 m2n2

(v) 2x2 – 5x – 3, 4x2 – 36
2x2 – 5x – 3 = (x – 3)(2x + 1)
4x2 – 36 = 4(x + 3)(x – 3)
L.C.M. = 4(x + 3)(x – 3)(2x + 1)
(vi) (2x2 – 3xy)2 = (x(2x – 3y))2
(4x – 6y)3 = (2(2x – 3y))3
8x3 – 27y3= (2x)3 – (3y)3
= (2x – 3y) (4x2 + 6xy + 9y2)
L.C.M. = 23 × x2 (2x – 3y)3 (4x2 + 6xy + 9y2)
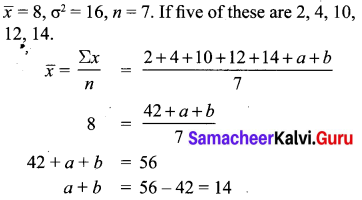 >
>