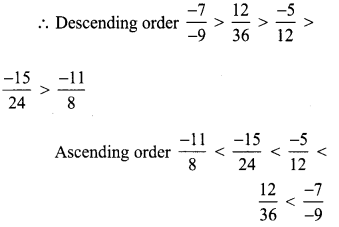
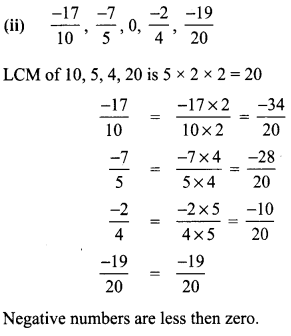
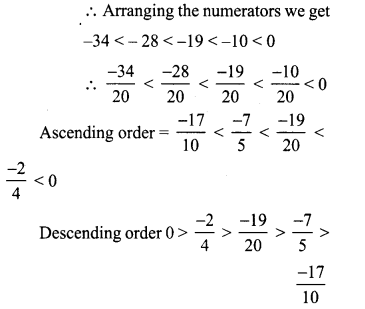
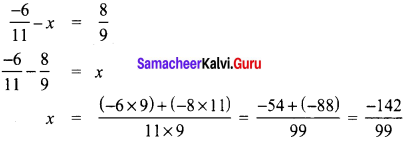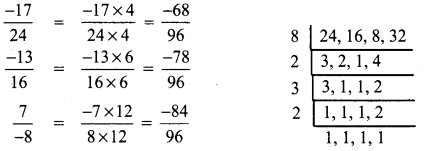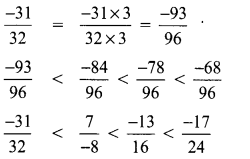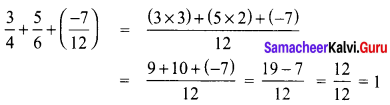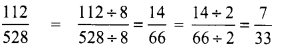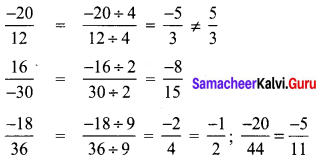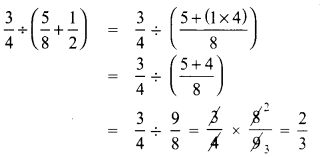Tamilnadu State Board New Syllabus Samacheer Kalvi 8th Tamil Book Solutions Guide Pdf Chapter 5.5 தொகைநிலை, தொகாநிலைத் தொடர்கள் Text Book Back Questions and Answers, Summary, Notes.
Tamilnadu Samacheer Kalvi 8th Tamil Solutions Chapter 5.5 தொகைநிலை, தொகாநிலைத் தொடர்கள்
கற்பவை கற்றபின்
Question 1.
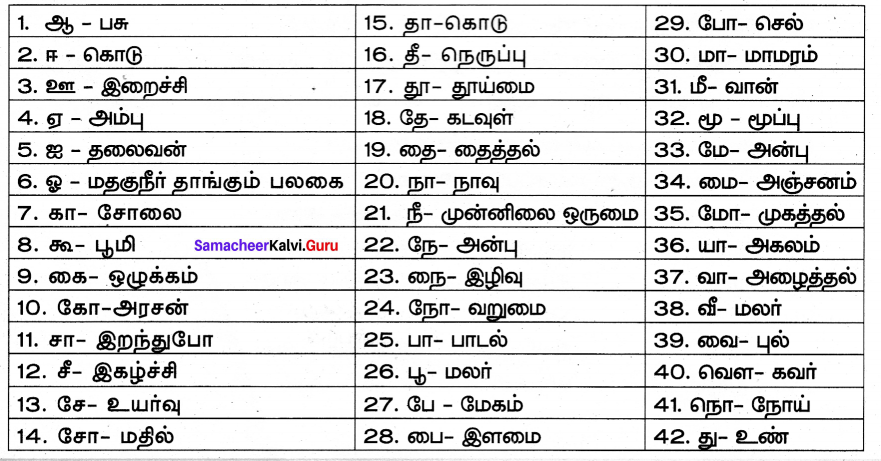
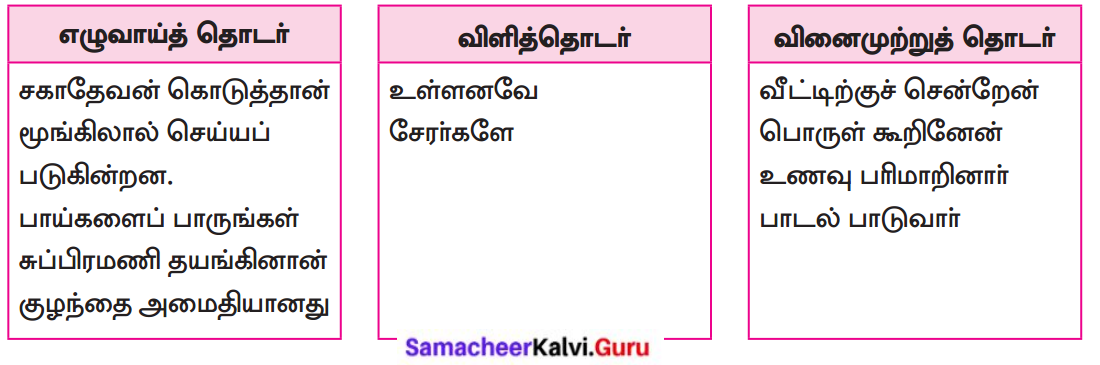
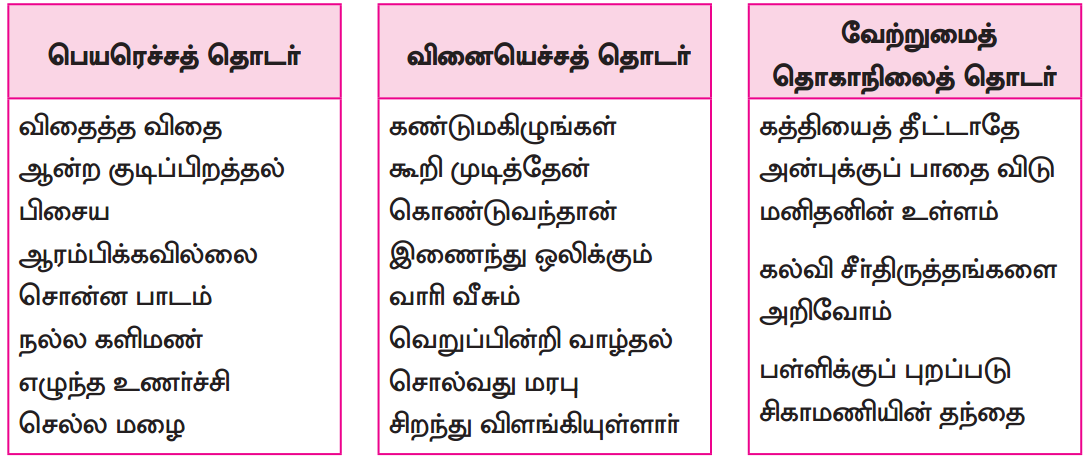
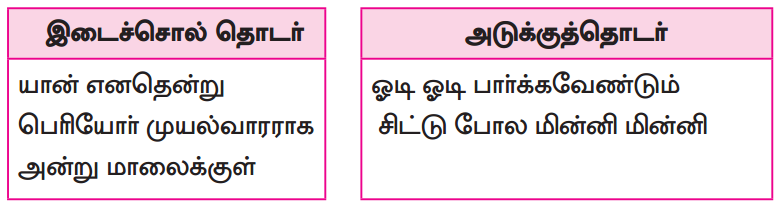
பாடப்பகுதியில் இடம்பெற்றுள்ள தொகைநிலைத் தொடர், தொகாநிலைத் தொடர்களைக் கண்டறிந்து தனித்தனியே தொகுக்க.
Answer:
தொகைநிலைத் தொடர்கள்
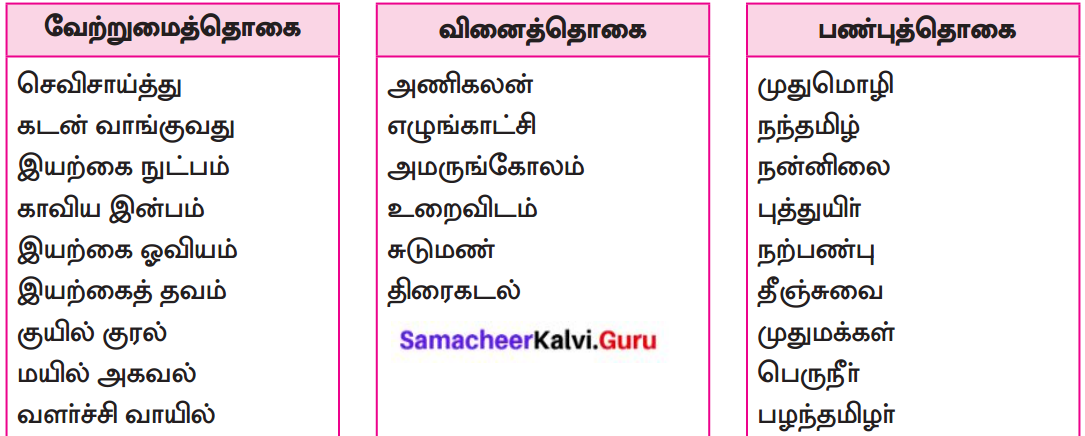
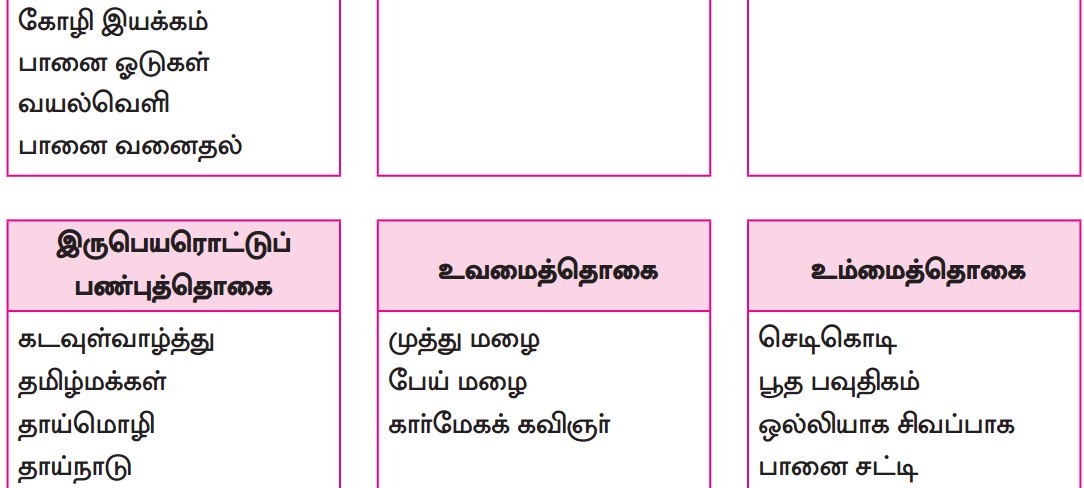

தொகா நிலைத்தொடர்கள்
பாடநூல் வினாக்கள்
சரியான விடையைத் தேர்ந்தெடுத்து எழுதுக.
Question 1.
சொற்களுக்கு இடையே வேற்றுமை உருபு மறைந்து வருவது ……………………….
அ) வேற்றுமைத் தொகை
ஆ) உம்மைத் தொகை
இ) உவமைத் தொகை
ஈ) அன்மொழித் தொகை
Answer:
அ) வேற்றுமைத் தொகை
Question 2.
‘செம்மரம்’ என்னும் சொல் …………………. த்தொகை.
அ) வினை
ஆ) பண்பு
இ) அன்மொழி
ஈ) உம்மை
Answer:
ஆ) பண்பு
Question 3.
‘கண்ணா வா!’ – என்பது ……………….. த் தொடர்.
அ) எழுவாய்
ஆ) விளி
இ) வினைமுற்று
ஈ) வேற்றுமை
Answer:
ஆ) விளி
பொருத்துக
1. பெயரெச்சத் தொடர் – அ) கார்குழலி படித்தாள்.
2. வினையெச்சத் தொடர் – ஆ) புலவரே வருக.
3. வினைமுற்றுத் தொடர் – இ) பாடி முடித்தான்.
4. எழுவாய்த் தொடர் – ஈ) எழுதிய பாடல்.
5. விளித் தொடர் – உ) வென்றான் சோழன்.
Answer:
1. ஈ
2. இ
3. உ
4. அ
5. ஆ
சிறுவினா
Question 1.
தொகைநிலைத் தொடர்கள் எத்தனை வகைப்படும்? அவை யாவை?
Answer:
தொகைநிலைத் தொடர்கள் ஆறு வகைப்படும். அவையாவன:
- வேற்றுமைத்தொகை
- உவமைத்தொகை
- வினைத்தொகை
- உம்மைத்தொகை
- பண்புத்தொகை
- அன்மொழித்தொகை
Question 2.
இரவு பகல் என்பது எவ்வகைத் தொடர் என விளக்குக.
Answer:
- ‘இரவு பகல்’ இத்தொடர், ‘இரவும் பகலும்’ என விரிந்து பொருள் தருகின்றது.
- இதில் சொல்லின் இடையிலும் இறுதியிலும் `உம்’ என்னும் இடைச் சொல் மறைந்து நின்று பொருள் தருகிறது.
- இவ்வாறு சொற்களுக்கு இடையிலும் இறுதியிலும் ‘உம்’ என்னும் இடைச் சொல் மறைந்து நின்று பொருள் தருவதை உம்மைத்தொகை என்பர்.
Question 3.
அன்மொழித்தொகையை எடுத்துக்காட்டுடன் விளக்குக.
Answer:
வேற்றுமை, வினை, பண்பு, உவமை, உம்மை ஆகிய தொகைநிலைத் தொடர்களில் அவை அல்லாத வேறு பிற சொற்களும் மறைந்து வருவது அன்மொழித்தொகை எனப்படும்.
சான்று : பொற்றொடி வந்தாள்.
இத்தொடர் ‘பொன்னாலாகிய வளையலை அணிந்த பெண் வந்தாள்’ என்னும் பொருள் தருகிறது. இதில் ‘ஆல்’ என்னும் மூன்றாம் வேற்றுமை உருபும் ‘ஆகிய என்னும் அதன் பயனும் மறைந்து வந்துள்ளது. ‘வந்தாள்’ என்னும் சொல்லால் பெண் என்பதையும் குறிப்பதால், இது மூன்றாம் வேற்றுமைப் புறத்துப் பிறந்த அன்மொழித் தொகை ஆகும்.
மொழியை ஆள்வோம்
கீழ்க்காணும் தலைப்பில் இரண்டு நிமிடம் பேசுக.
1. கைத்தொழில் ஒன்றைக் கற்றுக்கொள்
பெருமை மிகுந்த சான்றோர் சபைக்கு என் முதற்கண் வணக்கத்தைத் தெரிவித்துக் கொள்கின்றேன். இப்பெருமைமிகு சபையில் நான் பேச எடுத்துக் கொண்ட தலைப்பு கைத்தொழில் ஒன்றைக் கற்றுக்கொள் என்பதாகும்.
ஏட்டுச் சுரைக்காய் கறிக்கு உதவாது’ என்பார்கள். அதைப்போல ஏட்டுப் படிப்பு படித்தவர்களுக்கு எந்த வேலையும் கிடைப்பதாகத் தெரியவில்லை.
படித்தாலும், படித்துப் பட்டம் பெற்றாலும் கைத்தொழில் ஒன்றையும் நாம் கூடவே, சேர்த்துக் கற்றுக்கொள்ள வேண்டும். படிப்புக்கேற்ற வேலை கிடைக்கும் வரை அந்த வேலைக்காகக் காத்திராமல் கற்ற கைத்தொழில் நமக்கு மிகவும் பயன்படும்.
தையல், ஓவியம், மரவேலை, மின்னணுச் சாதனங்கள் பழுதுபார்ப்பு, தட்டச்சு, கணிப்பொறி, கூடை பின்னுதல், அலங்காரப் பொருட்கள் செய்தல் இவற்றைப் பொழுதுபோக்கிற்காக நாம் பள்ளியில் கற்றாலும், அங்கு ஆழமாக ஆழ்ந்து கற்க வேண்டும். அதுவேதான், பிற்காலத்தில் படிப்புக்கு ஏற்ற வேலை கிடைக்கும் வரையில் நமக்கு நல்ல சம்பாத்தியத்தைக் கொடுக்கும்.
ஏட்டுக்கல்வி கைவிட்டாலும், கைத்தொழில் கல்வி உன்னைக் கைவிடாது என்பதைப் புரிந்து கொள்ள வேண்டும். எனவே, இளைஞர்களாகிய நாம் கைத்தொழில் ஒன்றைக் கற்றுக் கொள்ள வேண்டும் என்று சொல்லி என்னுடைய உரையை நிறைவு செய்கின்றேன்.
2. இதயம் கவரும் இசை
என்னை ஈன்ற தாய் மொழிக்கும், இந்தச் சான்றோர் பேரவைக்கும் முதற்கண் வணக்கத்தைத் தெரிவித்துக்கொண்டு, இதயம் கவரும் இசை என்ற நல்லதொரு தலைப்பில் சில நிமிடங்கள் பேசுகின்றேன். ஒவ்வொருநாளும் ஒவ்வொரு மனிதனுக்கும் ஒவ்வொரு பிரச்சினைகள் இருக்கத்தான் செய்கின்றன. துன்பங்கள் நம்மைத் துரத்தும் போது மன அமைதி தானாக தேடி வருவதில்லை . இசையின் பக்கம் நாம் தான் ஓடி வர – வேண்டும். புல்லாங்குழல் இசையும், வீணை இசையும், நாத முழக்கமும், மத்தளம் இசையும் மனதைப் பண்படுத்தும். இசைக்கச்சேரி கேட்கும் போது இதயமெல்லாம் மென்மையாகிவிடும்.
சங்க காலத்தில் தலைவன் ஒருவன் கள் உண்ட மயக்கத்தில் படுத்து கிடக்கின்றான். தொலைவில் தலைவிதினையைக் காயவைத்துக் கொண்டிருக்கின்றாள். தலைவன் படுத்து இருந்த இடத்தை நோக்கி மத யானை ஒன்று ஓடி வருகின்றது .ஐயோ! தலைவனுக்கு என்ன ஆகுமோ? என்று கவலைப்படாமல், தலைவி அருகிலிருந்த யாழை எடுத்து மீட்டினாள். மதம் பிடித்த யானை யாழ் இசையில் மயங்கி தலைவனை மிதிக்காமல் தெளிந்து சென்றதாம். இசை உயிரையும் காப்பாற்றும்.
குழந்தை பிறந்ததும் தாலாட்டி இசை செய்தான், அவன் வளர்ந்து திருமணம் ஆகும் போதும் மங்கள இசைதான். இப்படி இசை வாழ்வில் எத்தனையோ இடத்திலும் இடம் பெற்றிருக்கிறது. நம் வாழ்க்கையில் ‘இதயம் கவரும் இசை அனைவரையும் கவரும் தசை’ என்று சொல்லி என் உரையை நிறைவு செய்கிறேன்.
நன்றி! வணக்கம்!!
சொல்லக் கேட்டு எழுதுக.
முல்லை நில மக்களாகிய ஆயர்கள் குழல் ஊதுவதில் வல்லவர்கள். இதனைச் சம்பந்தர் திருப்பதிகத்தில் அமைந்த நிகழ்ச்சி ஒன்று விளக்குகிறது. திருவண்ணாமலைச் சாரலில் ஆயர் ஒருவர் ஆநிரைகளையும் எருமையினங்களையும் மேய்த்துக் கொண்டிருந்தார். மாலையில் அவற்றை எல்லாம் ஒன்று திரட்டினார்.
அப்போது எருமை ஒன்று காணாமல் போனதை அறிந்தார். தன் கையிலிருந்த குழலை எடுத்து இனிய இசையை எழுப்பினார். இன்னிசை கேட்ட எருமை அவரை வந்தடைந்தது. இவ்வாறு ஆயர்களின் இசைத் திறத்தைத் திருப்பதிகம் விளக்குகிறது.
கோடிட்ட இடங்களில் பொருத்தமான சொல்லுருபுகளை இட்டு நிரப்புக.
(கொண்டு, இருந்து, உடைய, காட்டிலும், ஆக, நின்று, உடன், விட, பொருட்டு)
1. இடி ………………….. மழை வந்தது.
2. மலர்விழி தேர்வின் …………………. ஆயத்தமானாள்.
3. அருவி மலையில் …………………. வீழ்ந்தது.
4. தமிழைக் ……………….. சுவையான மொழியுண்டோ !
5. யாழ், தமிழர் …………………….. இசைக் கருவிகளுள் ஒன்று.
Answer:
1. உடன்
2. பொருட்டு
3. இருந்து
4. காட்டிலும்
5. உடைய
பின்வரும் இசைக்கருவிகளின் பெயர்களை அகர வரிசைப்படுத்துக.
படகம், தவில், கணப்பறை, பேரியாழ், உறுமி, உடுக்கை, தவண்டை , பிடில், நாகசுவரம், மகுடி.
Answer:
உடுக்கை, உறுமி, கணப்பறை, தவண்டை , தவில், நாகசுவரம், படகம், பிடில், பேரியாழ், மகுடி.
பின்வரும் இணைச்சொற்களை வகைப்படுத்துக.
உற்றார் உறவினர், விருப்பு வெறுப்பு, காலைமாலை, கன்னங்கரேல், ஆடல்பாடல், வாடிவதங்கி, பட்டிதொட்டி, உள்ளும் புறமும், மேடுபள்ளம், நட்ட நடுவில்.

சரியான இணைச்சொற்களை இட்டு நிரப்புக.
(மேடுபள்ளம், ஈடுஇணை, கல்விகேள்வி, போற்றிப்புகழப்பட, வாழ்வு தாழ்வு, ஆடிஅசைந்து)
1. சான்றோர் எனப்படுபவர் …………………………. களில் சிறந்தவர் ஆவார்.
2. ஆற்று வெள்ளம் …………………… பாராமல் ஓடியது.
3. இசைக்கலைஞர்கள் …………………. வேண்டியவர்கள்.
4. தமிழ் இலக்கியங்களின் பெருமைக்கு …………………….. இல்லை.
5. திருவிழாவில் யானை ………………. வந்தது.
Answer:
1. கல்விகேள்வி
2. மேடுபள்ளம்
3. போற்றிப்புகழப்பட
4. ஈடுஇணை
5. ஆடி அசைந்து
கடிதம் எழுதுக
இருப்பிடச் சான்று வேண்டி வட்டாட்சியருக்கு விண்ணப்பம் எழுதுக.
அனுப்புநர்
சா. சுந்தர்,
த/பெ. ஆ. சங்கர்
34, குறிஞ்சி நகர்,
ஈரோடு – 638 001.
பெறுநர்
உயர்திரு. வட்டாட்சியர் அவர்கள்,
வட்டாட்சியர் அலுவலகம்,
ஈரோடு.
மதிப்புக்குரிய அய்யா,
பொருள் : இருப்பிடச் சான்றிதழ் வேண்டுதல் சார்பாக. வணக்கம்.
ஈரோடு, அரசு உயர்நிலைப் பள்ளியில் எட்டாம் வகுப்பு படிக்கின்றேன். 34, குறிஞ்சி நகர், ஈரோடு – 638 001 என்ற முகவரியில் பத்து ஆண்டுகளாக நாங்கள் வசித்து வருகின்றோம். அரசின் கல்வி உதவித்தொகைக்கு விண்ணப்பிக்க இருப்பிடச் சான்றிதழ் தேவைப்படுகின்றது. இத்துடன் குடும்ப அட்டை நகலும் ஆதார் அட்டை நகலும் இணைத்துள்ளேன். ஆகவே, எனக்கு இருப்பிடச் சான்றிதழ் வழங்கும்படி பணிவுடன் கேட்டுக் கொள்கிறேன்.
நன்றி!
இடம் : ஈரோடு
நாள் : 25.06.2020
இப்படிக்கு,
தங்கள் உண்மையுள்ள,
சா. சுந்தர்.
உறைமேல் முகவரி:
பெறுநர்
உயர்திரு. வட்டாட்சியர் அவர்கள்,
வட்டாட்சியர் அலுவலகம்,
ஈரோடு.
மொழியோடு விளையாடு
குறுக்கெழுத்துப்புதிர்.
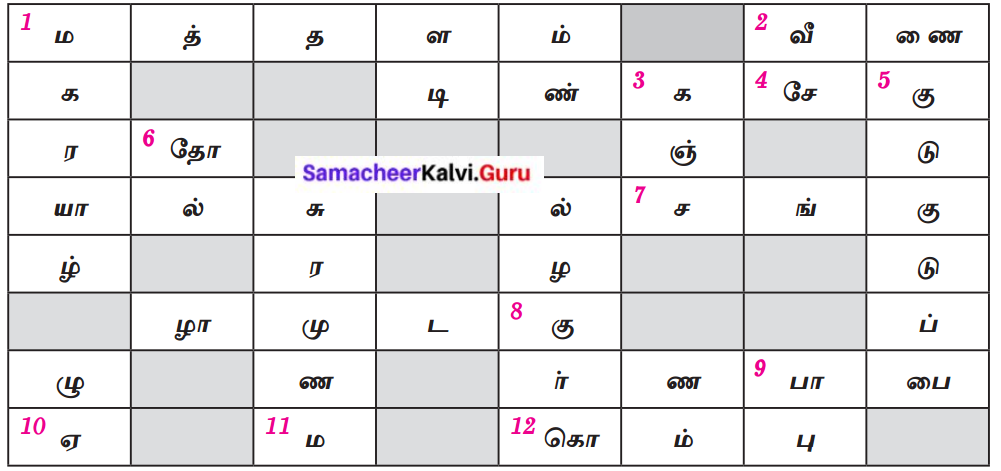
இடமிருந்து வலம் :
1. முதற்கருவி எனப் பெயர் பெற்றது.
2. யாழிலிருந்து உருவான பிற்காலக் கருவி
7. இயற்கைக் கருவி
12. விலங்கின் உறுப்பைப் பெயராகக் கொண்ட கருவி
வலமிருந்து இடம் :
4. வட்டமான மணி போன்ற கருவி
8. ஐந்து வாய்களைக் கொண்ட கருவி
9. இசைக்கருவிகளை இசைத்துப் பாடல் பாடுவோர்
மேலிருந்து கீழ் :
1. 19 நரம்புகளைக் கொண்ட யாழ்
3. ஒன்றோடு ஒன்று மோதி இசைக்கப்படுபவை
5. சிறிய வகை உடுக்கை
6. பறை ஒரு ……………… கருவி
கீழிருந்து மேல் :
8. மூங்கிலால் செய்யப்படும் காற்றுக்கருவி
10. வீணையில் உள்ள நரம்புகளின் எண்ணிக்கை
11. திருமணத்தின்போது கொட்டும் முரசு
நிற்க அதற்குத் தக
என் பொறுப்புகள்:
1. கைவினைக்கலைகளுள் ஒன்றைக் கற்றுக்கொள்வேன்.
2. இசைக் கலையை வளர்த்த சான்றோர்களைப் பற்றி அறிந்து போற்றுவேன்.
கலைச்சொல் அறிவோம்
1. கைவினைப் பொருள்கள் – Crafts
2. புல்லாங்குழல் – Flute
3. முரசு – Drum .
4. கூடைமுடைதல் – Basketry
5. பின்னுதல் – Knitting
6. கொம்பு – Horn
7. கைவினைஞர் – Artisan
8. சடங்கு – Rite
இணையத்தில் காண்க
இசையின் வகைப்பாடுகள் பற்றிய செய்திகளை இணையத்தில் தேடி எழுதுக.


கூடுதல் வினாக்கள்
சரியான விடையைத் தேர்ந்தெடுத்து எழுதுக..
Question 1.
திருவாசகம் படித்தாள் – இதில் மறைந்து வரும் வேற்றுமை உருபு …………………..
அ) இரண்டாம் வேற்றுமை உருபு
ஆ) மூன்றாம் வேற்றுமை உருபு
இ) நான்காம் வேற்றுமை உருபு
ஈ) ஐந்தாம் வேற்றுமை உருபு
Answer:
அ) இரண்டாம் வேற்றுமை உருபு
Question 2.
கம்பர் பாடல் – இதில் இடம்பெற்றுள்ள வேற்றுமையுருபு ……………………
அ) கு
ஆ) இன்
இ) அது
ஈ) கண்
Answer:
இ) அது
Question 3.
காலம் கரந்த பெயரெச்சம் ………………..
அ) வினைத்தொகை
ஆ) பண்புத்தொகை
இ) வேற்றுமைத்தொகை
ஈ) உவமைத்தொகை
Answer:
அ) வினைத்தொகை
Question 4.
ஆடுகொடி, வளர்தமிழ் – ஆகியன …………………. க்குச் சான்றுகள்.
அ) வேற்றுமைத்தொகை
ஆ) உம்மைத்தொகை
இ) உவமைத்தொகை
ஈ) வினைத்தொகை
Answer:
ஈ) வினைத்தொகை
Question 5.
வெண்ணிலவு, கருங்குவளை ஆகியன ………………….. க்குச் சான்றுகளாகும்.
அ) வினைத்தொகை
ஆ) பண்புத்தொகை
இ) உவமைத்தொகை
ஈ) உம்மைத்தொகை
Answer:
ஆ) பண்புத்தொகை
Question 6.
இருபெயரொட்டு பண்புத்தொகைக்குச் சான்றாக அமையும் ஒன்றினைத் தேர்வு செய்க.
அ) மலர்விழி
ஆ) இரவுபகல்
இ) பொற்றொடி வந்தாள்
ஈ) பனைமரம்
Answer:
ஈ) பனைமரம்
Question 7.
இரவுபகல், தாய்தந்தை ஆகியன ………………………. க்குச் சான்றாகும்.
அ) அன்மொழித்தொகை
ஆ) உவமைத்தொகை
இ) உம்மைத்தொகை
ஈ) இருபெயரொட்டுப் பண்புத்தொகை
Answer:
இ) உம்மைத்தொகை
Question 8.
தொகாநிலைத் தொடர் வகைகள்
அ) 6
ஆ) 8
இ) 9
ஈ) 3
Answer:
இ) 9
Question 9.
எழுவாய்த் தொடர் அமையும் சான்றினைத் தேர்ந்தெடுக்க.
அ) மல்லிகை மலர்ந்தது
ஆ) நண்பா படி
இ) சென்றனர் வீரர்
ஈ) வரைந்த ஓவியம்
Answer:
அ) மல்லிகை மலர்ந்தது
Question 10.
‘நண்பா படி’ என்பது ……………………
அ) எழுவாய்த் தொடர்
ஆ) விளித் தொடர்
இ) வினைமுற்றுத்தொடர்
ஈ) பெயரெச்சத் தொடர்
Answer:
ஆ) விளித்தொடர்
குறுவினா
Question 1.
வேற்றுமைத் தொகை என்றால் என்ன? சான்று தருக.
Answer:
- இருசொற்களுக்கு இடையில் வேற்றுமை உருபு மறைந்துவந்து பொருள் தந்தால், அதனை வேற்றுமைத் தொகை என்பர்.
- சான்று: திருவாசகம் (ஐ)படித்தான். (இரண்டாம் வேற்றுமைத் தொகை)
Question 2.
உருபும் பயனும் உடன்தொக்கத் தொகை என்றால் என்ன? சான்று தருக.
Answer:
- ஒரு தொடரில் வேற்றுமை உருபும் அதன் பொருளை விளக்கும் சொல்லும் மறைந்து வருவது உருபும் பயனும் உடன் தொக்கத் தொகை எனப்படும்.
- சான்று: பால் குடம்.
- பாலைக் கொண்ட குடம்’ இரண்டாம் வேற்றுமை உருபும் பயனும் உடன் தொக்கத் தொகை.
Question 3.
வினைத்தொகையை சான்றுடன் விளக்குக.
Answer:
- காலம் காட்டும் இடைநிலையும், பெயரெச்சவிகுதியும் மறைந்து வரும் பெயரெச்சம் வினைத்தொகை என்பர்.
- சான்று: வளர்தமிழ். இதில் காலம் காட்டும் இடைநிலைகள் தொக்கி வந்துள்ளன.
- வளர்ந்த தமிழ், வளர்கின்ற தமிழ், வளரும் தமிழ் எனவும் முக்காலத்திற்கும் பொருந்தும் படியாகப் பொருள் தருகின்றன. எனவே, இது வினைத்தொகை ஆகும்.
Question 4.
பண்புத்தொகை என்றால் என்ன? சான்று தருக.
Answer:
- பண்புப்பெயருக்கும் அது தழுவி நிற்கும் பெயர்ச்சொல்லுக்கும் இடையே ‘ஆன்’, ‘ஆகிய’ என்னும் பண்பு உருபுகள் மறைந்து வருவது பண்புத்தொகை எனப்படும்.
- சான்று: வெண்ணிலவு, கருங்குவளை.
Question 5.
‘மலர்விழி’ என்னும் சான்று அமையும் தொகை யாது? விளக்கு.
Answer:
‘மலர்விழி’ என்பது உவமைத்தொகை. மலர் போன்ற விழி என்ற பொருளைத் தருகிறது. ‘மலர்’ என்பது உவமை. ‘விழி’ என்பது உவமேயம். ‘போன்ற’ என்பது உவம உருபு. உவமைக்கும் உவமேயத்துக்கும் இடையில் உவம உருபு மறைந்து வந்தால் அது உவமைத்தொகை எனப்படும்.
Question 6.
எண்ணும்மை என்றால் என்ன? சான்று தருக.
Answer:
ஒன்றுக்கு மேற்பட்ட சொற்களில் ‘உம்’ என்னும் உருபு வெளிப்பட வருவது எண்ணும்மை ஆகும். சான்று: இரவும் பகலும், பசுவும் கன்றும்
சிறு வினா
Question 1.
தொகாநிலைத் தொடர் என்றால் என்ன? அதன் வகைகளை எழுதுக.
Answer:
(i) ஒரு தொடரில் இரு சொற்கள் வந்து அவற்றின் இடையில் சொல்லுருபு மறையாமல் நின்று பொருள் தந்தால் அதனை தொகாநிலைத்தொடர் என்பர்.
(ii) தொகாநிலைத்தொடர் ஒன்பது வகைப்படும். எழுவாய்த்தொடர், விளித்தொடர், வினைமுற்றுத் தொடர், பெயரெச்சத் தொடர், வினையெச்சத் தொடர், வேற்றுமைத் தொகாநிலைத் தொடர், இடைச்சொல் தொடர், உரிச்சொல் தொடர், அடுக்குத்தொடர்.