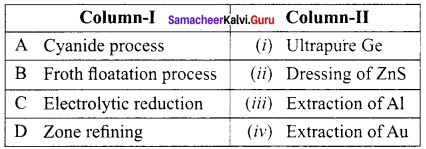
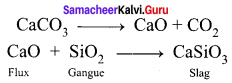
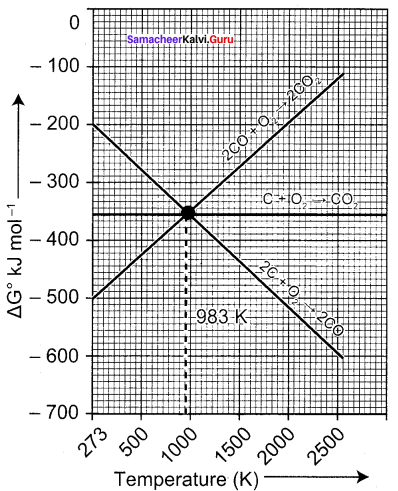
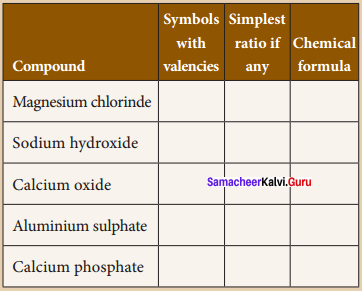
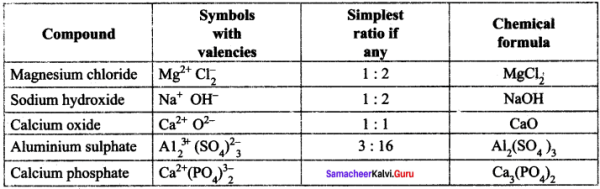
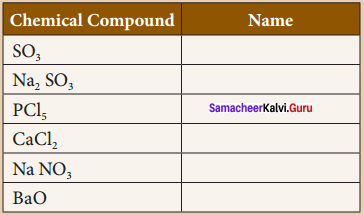
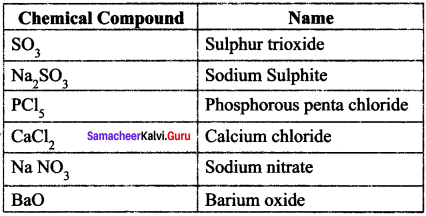
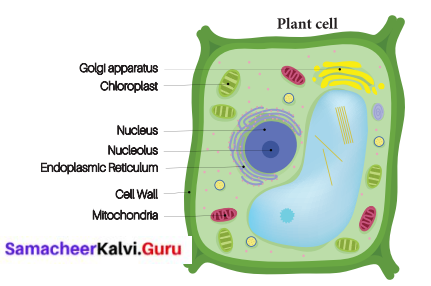
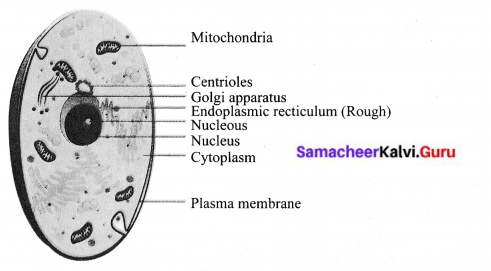
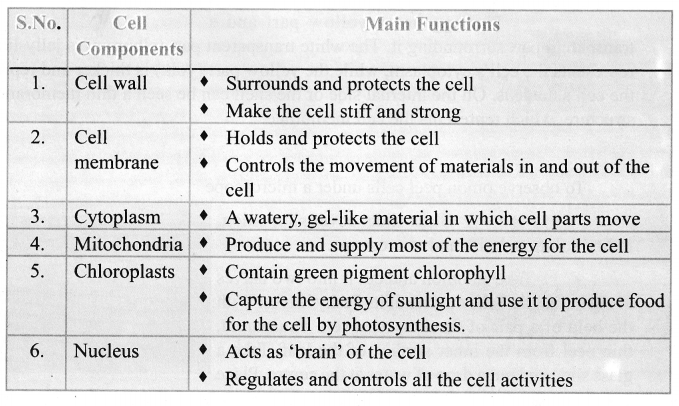
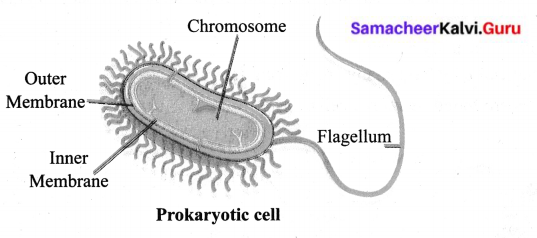
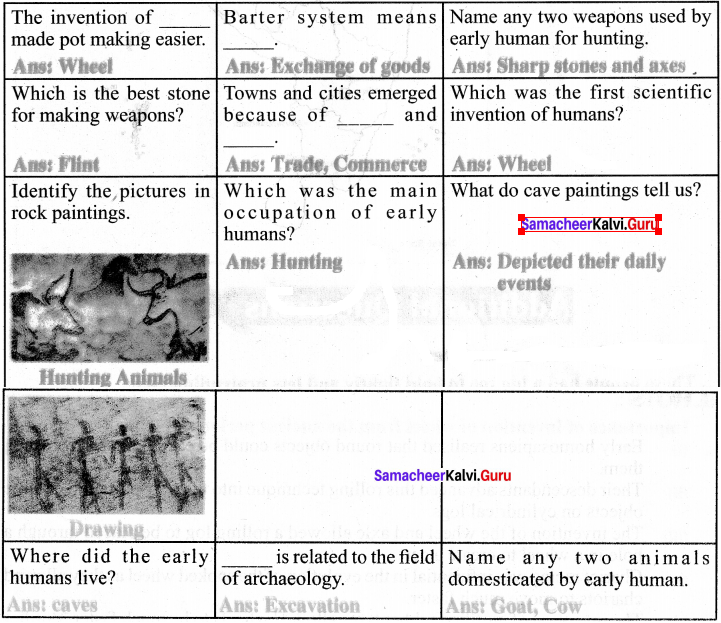
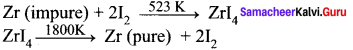You can Download Samacheer Kalvi 9th Science Book Solutions Guide Pdf, Tamilnadu State Board help you to revise the complete Syllabus and score more marks in your examinations.
Tamilnadu Samacheer Kalvi 9th Science Solutions Chapter 23 Economic Biology
Samacheer Kalvi 9th Science Economic Biology Textbook Exercises
I. Choose the correct answer.
Economic Biology 9th Class Question 1.
The production and management of fish is called ……………..
(a) Pisciculture
(b) Sericulture
(c) Aquaculture
(d) Monoculture
Answer:
(a) Pisciculture
Chapter 23 Economic Biology Question 2.
Which one of the following is not an exotic breed of cow?
(a) Jersey
(b) Holstein-Friesan
(c) Sahiwal
(d) Brown Swiss
Answer:
(c) Sahiwal
9th Science Economics Biology Question 3.
Which one of the following is an Italian species of honey bee?
(a) Apis mellifera
(b) Apis dorsata
(c) Apis florae
(d) Apis Cerana
Answer:
(a) Apis mellifera
Samacheer Kalvi 9th Science Question 4.
Which one of the following is not an Indian major carp?
(a) Rohu
(b) Catla
(c) Mrigal
(d) Singhara
Answer:
(d) Singhara
Samacheer Kalvi Guru 9th Science Question 5.
Drones in the honey bee colony are formed from
(a) unfertilized egg
(b) fertilized egg
(c) parthenogenesis
(d) both b and c
Answer:
(b) fertilized egg
Samacheer Kalvi 9th Science Solutions Question 6.
Which of the following is an high milk yielding variety of cow?
(a) Holstein- Friesan
(b) Dorset
(c) Sahiwal
(d) Red Sindhi
Answer:
(a) Holstein- Friesan
9th Science Samacheer Kalvi Question 7.
Which Indian variety of honey bee is commonly used for apiculture?
(a) Apis dorsata
(b) Apis florea
(c) Apis mellifera
(d) Apis indica
Answer:
(d) Apis indica
Samacheer Kalvi 9th Science Guide Question 8.
………………. is the method of growing plants without soil.
(a) Horticulture
(b) Hydroponics
(c) Pomology
(d) None of these
Answer:
(b) Hydroponics
Samacheer Kalvi 9th Science Solution Question 9.
The symbiotic association of fungi and vascular plants is …………………….
(a) Lichen
(b) Rhizobium
(c) Mycorhizae
(d) Azotobacter
Answer:
(c) Mycorhizae
9th Science Solutions Samacheer Kalvi Question 10.
The plant body of mushroom is …………………….
(a) Spawn
(b) Mycelium
(c) Leaf
(d) All of these
Answer:
(c) Leaf
II. Fill in the blanks.
Samacheer Kalvi Class 9 Science Solutions Question 11.
Quinine drug is obtained from ……………….
Answer:
Cinchona officinalis
Samacheer Kalvi 9th Biology Book Pdf Question 12.
Carica papaya leaf can cure …………….. disease.
Answer:
Apiculture
Samacheer Kalvi 9th Science Practical Question 13.
Vermicompost is a type of soil made by ………………. and microorganisms.
Answer:
earthworms
Samacheer Kalvi Biology Question 14
……………….. refers to the culture of prawns, pearl and edible oysters.
Answer:
Aquaculture
Samacheer Kalvi 9th Science Book Solutions Question 15.
The largest member in a honey bee haive is the …………………..
Answer:
F
Samacheer Kalvi 9th Standard Science Question 16
……………… is a preservative in honey.
Answer:
Formic acid
Science Solution Class 9 Samacheer Kalvi Question 17
…………………. is the method of culturing different variety of fish in a water body.
Answer:
Polyculture
III. State whether true or false, If false, correct the given statement.
- Mycorrhiza is an algae – False.
Correct Statement: Mycorrhiza is a fungi - Milch animals are used in agriculture and transport – False.
Correct Statement: Milch animals are domesticated for obtaining only milk. - Apisflorea is a rock bee – False.
Correct Statement: Apis Florea is a little bee - Ongole is an exotic breed of cattle – False.
Correct Statement: Ongole is a dual-purpose breed of cattle - Sheep manure contains high nutrients than farmyard manure – True.
IV. Differentiate the following
9th Samacheer Kalvi Science Question 1.
Exotic breed and Indigenous breed.
Answer:
| Exotic breed | Indigenous breed |
| Exotic breeds are imported from foreign countries | Indigenous breed are native to India |
| These foreign breeds are selected for long lactation periods. | These local breed show excellent resistance to diseases. |
| Example: Jersey, Brown Swiss and Holstein-Friesian | Example: Sahiwal, Red Sindhi, Deoni and Gir. |
Samacheer Kalvi 9 Science Question 2.
Pollen and Nectar
Answer:
| Pollen | Nectar |
| Pollen is a fine to a coarse powdery substance comprising pollen grains which are male microgametophytes of seed plants, which produce male gametes | It is a sweet viscous secretion secreted by the flower of plants. |
Science 9th Samacheer Kalvi Question 3.
Shrimp and Prawn
Answer:
| Shrimp | Prawn |
| Shrimp has lamellar gills. | Prawns have branching gills. |
| Shrimp have claws on two of their five pairs of legs. | Prawns have claws on three of their five pairs of legs. |
Samacheer Kalvi Guru 9th Science Solutions Question 4.
Farmyard manure and Sheep manure
Answer:
| Farmyard manure | Sheep manure |
| Well decomposed farm yard manure contains 0.5% Nitrogen, 0.2% available phosphate and 0.5% available potash. | It contains 3% Nitrogen, 1% phosphorus pentoxide and 2% potassium oxide. |
V. Match the following.
| Column A | . Column B | |
| 1. | Lobsters | (a) Marine fish |
| 2. | Catla | (.b) Pearl |
| 3. | Sea bass | (c) Shell fish |
| 4. | Oysters | (d) Paddy |
| 5 | Pokkali | (.e) Fin fish |
| 6 | Pleurotus sps | (J) Psoriosis |
| 7 | Sarpagandha | (g) Oyster mushroom |
| 8 | Olericulture | (h) Reserpine |
| 9 | Wrighta tinctoria | (0 Vegetable farming |
Answer:
- (c) Shellfish
- (e) Finfish
- (a) Marine fish
- (b) Pearl
- (d) Paddy
- (g) Oyster mushroom
- (h) Reserpine
- (i) Vegetable farming
- (f) Psoriasis
VI. Answer in brief.
Samacheerkalvi.Guru 9th Science Question 1.
What are secondary metabolites?
Answer:
Most medicines are obtained either directly or indirectly from plants. All the major system of medicines such as Ayurveda, Yoga, Unani, Siddha, Homeopathy (AYUSH) use drugs obtained from plants and animals. These drugs from medicinal plants are called secondary metabolites.
Question 2.
What are the types of vegetable garden?
Answer:
Vegetable farming can be classified into:
- Kitchen or Nutrition gardening,
- Commercial gardening,
- Vegetable forcing.
Question 3.
Mention any two mushroom preservation methods.
Answer:
Drying and Vacuum Cooling are some methods used to preserve mushroom.+
Question 4.
Enumerate the advantages of vermicompost over chemical fertiliser.
Answer:
- It is a rich source of nutrients essential for plant growth. It makes the soil fertile.
- It improves soil structure, texture, aeration and water holding capacity and helps to prevent soil erosion.
- It contains valuable vitamins, enzymes and growth regulator substances for increasing growth, vigour and yield of plants.
- It enhances decomposition of organic matter in soil.
- Vermicompost is free from pathogens and toxic elements.
- Vermicompost is rich in beneficial microflora.
Question 5.
What are the species of earthworm used for vermiculture?
Answer:
Among the vast community of earthworms only very few species can be used for vermicompost production. They are Perionyx excavatus (Indian blueworm), Eisenia fetida (Red worms), Eudrilus eugeniae (African night crawler).
Question 6.
List the medicinal importance of honey.
Answer:
Uses of Honey
- Honey has an antiseptic and antibacterial property. It is a blood purifier.
- It helps in building up of haemoglobin content in the blood.
- It is used in Ayurvedic and Unani system of medicines.
- It prevents cough, cold, fever and relieves sore throat.
- It is a remedy for ulcers of tongue, stomach and intestine.
- It enhances digestion and appetite.
VII. Answer in detail.
Question 1.
Enumerate the advantage of hydroponics.
Answer:
Hydroponics was demonstrated by a German Botanist Julius Von Sachs in 1980.
Advantages
- Crops can be grown in places where the land is limited, doesn’t exist, or is heavily contaminated.
- The climate – temperature, humidity, light intensification, the composition of the air can be monitored.
- Conservation of water and nutrients.
- Controlled plant growth.
- No intrusion by weeds.
- Fewer pests & diseases
- Minimal use of insecticide or herbicides
- In deserts and Arctic regions hydroponics can be an effective alternative method.
- Hydroponics is successfully employed for the commercial production of seedless cucumber and tomato.
Question 2.
Define Mushroom culture. Explain the mushroom cultivation methods.
Answer:
Mushroom is a fungi belonging to basidiomycetes. It is rich in proteins, fibres, vitamins and minerals. Mushroom culture is the process of producing food, medicine, and other products by the cultivation of mushrooms.
Mushroom can be cultivated either on paddy straw or on wood log.
Major stages of mushroom cultivation are;
- Composting:
Compost is prepared by mixing paddy straw with number of organic materials like cow dung and inorganic fertilizers. It is kept at about 50°C for one week. - Spawning:
Spawn is the mushroom seed. It is prepared by growing fungal mycelium in grains under sterile conditions. Spawn is sown on compost. - Casing:
Compost is covered with a thin layer of soil. It gives support to the growing mushroom, provides humidity and helps regulate the temperature. - Pinning:
Mycelium starts to form little bud, which will develop into mushroom. Those little white buds are called pins. - Harvesting:
Mushroom grow better in 15°C – 23°C. They grow 3 cm in a week which is the normal size for harvesting. In the third week the first flush mushroom can be harvested.
Question 3.
What are the sources of organic resources for vermicomposting?
Answer:
Materials required for vermicomposting
Biologically degradable organic wastes are used as potential organic resources for vermicomposting. They are:
- Agricultural wastes (crop residue, vegetable waste, sugarcane trash)
- Crop residues (rice straw, tea wastes, cereal and pulse residues, rice husk, tobacco wastes, coir wastes)
- Leaf litter
- Fruit and vegetable wastes
- Animal wastes (cattle dung, poultry droppings, pig slurry, goat and sheep droppings)
- Biogas slurry
Question 4.
Give an account of different types of fish ponds used for rearing fishes.
Answer:
Types of ponds for fish culture
Fish farm requires different types of pond for the various developmental stages of fish growth. They are:
- Breeding pond: Healthy and sexually mature male and female fishes are collected and introduced in this pond for breeding. The eggs released by the female are fertilized by the sperm and fertilized eggs float in water as frothy mass.
- Hatchling pits: The fertilized eggs are transferred to hatching pits for hatching. Two types of hatching pits are hatcheries and hatching hapas.
- Nursery ponds: The hatchlings are transferred from hatching pits after 2 to 7 days. The hatchlings grow into fry and are cultured in these ponds for about 60 days with proper feeding till they reach 2 -2.5 cm in length.
- Rearing ponds: Rearing ponds are used to culture the fry. The fish fry are transferred from nursery pond to rearing ponds and are maintained for about three months till they reach 10 to 15 cm in length. In these rearing ponds the fry develops into fingerlings.
e) Stocking pond: The stocking pond is also called a culture pond or production pond. These ponds are used to rear fingerlings upto the marketable size. Before releasing the fingerlings, the pond is manured with organic manure and inorganic fertilizers.
Question 5.
Classify the different breeds of the cattle with suitable examples.
Answer:
Cattle breeds
The Indian cattle include cows and buffaloes. They are domesticated for milk, meat, leather and transportation. They belong to two different species, Bos indicus (Indian cows and bulls) and Bos bubalis (buffaloes). These cattle animals are reared for milk and farm labour. They are classified into three types:
- Dairy breeds,
- Draught (or) Draft breeds,
- Dual-purpose breeds
Dairy breeds:
Dairy animals are domesticated for obtaining milk. The cows (milk producing females) are high milk yielders (milch animals). The dairy breeds may be indigenous breeds (or) exotic breeds.
- Indigenous breeds are native of India. They include Sahiwal, Red Sindhi, Deoni and Gir. These cattle are well built with strong limbs, prominent hump and loose skin. These local breed animals show excellent resistant to diseases.
- The exotic breeds (Bos taurus) are imported from foreign countries. They include Jersey, Brown Swiss and Holstein-Friesian etc. These foreign breeds are selected for long lactation periods. The Indian (local) breeds and foreign breeds can be cross bred to produce animals with both desired qualities.
Draught (or) Draft breeds:
They are used for agricultural work, such as tilling, irrigation and carting. These include Amritmahal, Kangayam, Umblachery, Malvi, Siri and Hallikar breeds. Bullocks are good draft animals while the cows are poor milk yielders.
Dual-purpose breeds:
These breeds provide milk and they are useful for farm work. In India these breeds are favoured by farmers as the cows are fairly good milk yielders and bullocks are good for draught work. They includes Haryana, Ongole, Kankrej and Tharparkar.
Buffalo breeds:
In India, buffaloes are domesticated in great number. They are the main milk producers. The milk production of buffaloes is more than that of cows. Murrah, Mehsana and Surti are indigenous buffalo breeds which are good milk yielders.
VIII. Higher Order Thinking Skills.
Question 1.
Biomanuring plays an important role in agriculture. Justify
Answer:
Biomanure also knwon as organic manures, are predominantly derived from plant debris, animal faeces and microbes. They make the soil fertile by adding nutrients like nitrogen. They are eco-friendly. Biomanure is easy to generate and very economical. Some examples of biomanure are Animal manure, Vermicompost and Green Manure.
Question 2.
Each bee hive consists of hexagonal cells. Name the material in which the cell is formed and mention the significance of the hexagonal cells.
Answer:
The cell is formed in a sheet of wax. The hexagonal shape allows to hold the queen bee’s eggs and store the pollen and honey the worker bees bring to the hive.
Activity
Question 1.
Discuss in your class room about the importance of crop insurance to farmers.
Answer:
In agriculture there are risks beyond one’s control. Hence precautionary measures are to be considered to control damage faced by farmers. Farmers who take crop insurance protect their crop and families from unforeseen setbacks.
The advantages of crop insurance are,
- Stability in Income
- Minimal Debts
- Farmers can safely invest on new technological advancements to improve their crop production.
- Protection against loss of crops
- Provides Awareness on natural calamities and the preventive measures to be taken
Question 2.
Collect at least five medicinal plants from your locality. Identify the plant and try to find out its medicinal value.
Answer:
Students can perform this activity under the guidance of the class teacher.
Question 3.
Visit a fish farm during the breeding season near your locality and collect information.
Answer:
Students can perform this activity under the guidance of the class teacher.
Samacheer Kalvi 9th Science Economic Biology Additional Questions
I. Choose the correct answer.
Question 1.
………………. is a branch of agriculture that deals with cultivation of fruits, vegetables and
ornamental plants.
(a) Floriculture
(b) Horticulture
(c) Olericulture
(d) Moriculture
Answer:
(b) Horticulture
Question 2.
……………….. is growing of vegetables in small scale in households.
(a) Vegetable farming
(b) Flower farming
(c) Kitchen gardening
(d) Vegetable forcing
Answer:
(c) Kitchen gardening
Question 3.
Which one of the following is an African species of Honey bee?
(a) Apis florae
(b) Apis indica
(c) Apis mellifera
(d) Apis adamsoni
Answer:
(d) Apis adamsoni
Question 4.
Which one of the following is not an indegenous of cow?
(a) Sahiwal
(b) Jersey
(c) Red Sindhi
(d) Deoni
Answer:
(b) Jersey
Question 5.
……………… involves rising of cattle for milk production.
(a) Dairy farming
(b) Drying
(c) Freezing
(d) Canning
Answer:
(a) Dairy farming
Question 6.
………………. are low in fibre and contain high level of carbohydrates, protein and other nutrients.
(a) Cattle feed
(b) Roughage
(c) Concentrates
(d) Feed management
Answer:
(c) Concentrates
Question 7.
The ………………. contain the young stages of the honey bees and they are built in the centre
and lower part of the comb.
(a) brood cells
(b) storage cells
(c) drone chamber
(d) queen chamber
Answer:
(a) brood cells
Question 8.
………………….. feeds on the organic wastes.
(a) Bees
(b) Earthworms
(c) Prawns
(d) Cattle
Answer:
(b) Earthworms
II. Fill in the blanks.
- ………………. is the science of growing vegetables.
- Compost is a ……………….. as well as a fertilizer which is rich in nutrients.
- …………….. is a small bacterium that colonize the roots of leguminous plants to form root nodules.
- Application of ……………… has been found to increase yield of wheat, rice, maize and sorghum.
- Mycelium starts to form little bud which develops into a ……………..
- Compost for mushroom cultivation is prepared by mixing ………………… with number of organic materials like cow dung and inorganic fertilizers.
Answer:
- Olericulture
- soil conditioner
- Rhizobium
- Azotobacter
- mushroom
- paddy straw
III. State whether the following statements are true or false. If false, write the correct statement.
- Vermicomposting is the rearing of earthworms for the production of vermicompost – True
- Pasturage is the production of fruits – False.
Correct Statement: Pasturage is the availability of flowers to bees for nectar and pollen collection. - Binomial name of Nilavembu is Leucas aspera – False.
Correct Statement: Binomial name of Nilavembu is Andrographis paniculata. - Mariculture is the culture of fishes and another aquatic organism in marine water near the sea coast – True
- Operation flood programme is based on dairy commodity to increase milk supply in urban areas – True.
IV. Match column A with column B.
| S.No. | Column A | Column C | |
| 1. | Tulsi | a. | Wrightia tinctoria |
| 2. | Nannari | b | Cathyranthus roseus |
| 3. | Vepalai | c | Eucalyptus globulus |
| 4 | Cinjona maram | d | Hemidesmus indicus |
| 5 | Nithya kalyani | e | Cinchona officinalis |
| 6 | Thaila maram | f | Ocimum sanctum |
Answer:
| S.No. | Column A | Column C | |
| 1. | Tulsi | a. | Ocimum sanctum |
| 2. | Nannari | b | Hemidesmus indicus |
| 3. | Vepalai | c | Wrightia tinctoria |
| 4 | Cinjona maram | d | Cinchona officinalis |
| 5 | Nithya kalyani | e | Cathyranthus roseus |
| 6 | Thaila maram | f | Eucalyptus globulus |
V. Differentiate the following.
Question 1.
Marine water prawn culture and Freshwater prawn culture
Answer:
| Marine water prawn culture | Freshwater prawn culture |
| The rearing of marine penaied prawn is called marine prawn culture or shrimp culture. | The rearing of freshwater prawn is called fresh water prawn culture. |
Question 2.
Extensive fish culture and Intensive fish culture
Answer:
| Extensive fish culture | Intensive fish culture |
| Culture of fishes in large areas with low stocking density and natural feeding. | Culture of fishes in small areas with high stocking density and providing artificial feed to increase production. |
Question 3.
Storage cells and Brood cells
Answer:
| Storage cells | Brood cells |
| The storage cells contain honey and pollen. | The brood cells contain the young stages of the honey bees. |
| They are built in the margin and at the top of the comb. | They are built in the centre and the lower part of the comb. |
VI. Answer in brief.
Question 1.
Who are the Father of Indian medicines.
Answer:
| Ayurveda | Charaka Samhita |
| Yoga | Patanjali |
| Unani | Hippocrates |
| Siddha | Agasthya |
| Homeopathy | Samuel Hahnemann |
Question 2.
What is spawning?
Answer:
Spawn is the mushroom seed. It is prepared by growing fungal mycelium in grains under sterile conditions. Spawning is the sowing or planting of spawn on compost.
Question 3.
What are the types of aquaculture?
Answer:
Aquaculture is classified into;
- Freshwater aquaculture
- Brackish water aquaculture
- Marine water aquaculture (Mariculture)
Question 4.
What is aquaponics?
Answer:
Aquaponics is a system of a combination of conventional aquaculture with hydroponics in a symbiotic environment in which plants are fed with the aquatic animals excreta or wastes.
Question 5.
What is green manure? How is it beneficial?
Answer:
Green manure is obtained by collection and decomposition of green leaves, twigs of trees, shrubs and herbs growing in wastelands, field bunds etc. Green manure improves soil structure, increases water holding capacity and decreases soil loss by erosion. It also helps in reclamation of alkaline soils and reduces weed proliferation. It is a manure obtained from undecomposed green material derived from leguminous plants e.g. Sunhemp, Dhaincha, etc.
Question 6.
Write a note on nutritional value of fishes.
Answer:
Cultivable freshwater and marine food fishes are highly nutritious, rich source of animal proteins and are easily digestible. They are rich in essential amino acids such as lysine and methionine, minerals like calcium, phosphorus, iron, sodium, potassium and magnesium. Fat soluble vitamins A, D and water soluble B-complex vitamins like pyridoxine, cyanocobalamine and niacin ate found in fishes. Polyunsaturated fatty acid (PUFA) which are helpful in regulation of cholesterol are present in plenty in fishes and thus promote cardiac health.
VII. Answer in detail.
Question 1.
Explain hydroponics and give its importance.
Answer:
Hydroponics is the method of growing plants without soil, using mineral nutrient solutions in water. The containers are made of glass, metal or plastic. They range in size from small pots for individual plants to huge tank for large scale growing. It was demonstrated by a German Botanist Julius Von Sachs in 1980. Hydroponics is successfully employed for the commercial production of seedless cucumber and tomato.
Plants are suspended with their roots submerged in water that contain plant nutrients. The roots absorb water and nutrients, but do not perform the anchoring function. Therefore, the plants must be mechanically supported from above.
Importance of hydroponics
- Conservation of water and nutrients.
- Controlled plant growth.
- In deserts and Arctic regions hydroponics can be an effective alternative method.
Question 2.
What are the types of honey bees found in a colony?
Answer:
There are three types of individuals in a colony namely the Queen bee, the drones and the worker bees.
- Queen Bee: The queen is the largest member and the fertile female of the colony. They are formed from fertile eggs. The queen is responsible for laying eggs in a colony. The life span of the queen bee is 3-4 years.
- Drones: They are the fertile males. They develop from unfertilized eggs. They are larger than the workers and smaller than the queens. Their main function is to fertilize the eggs produced by the queen.
- Worker Bees: They are sterile female bees and are the smallest members of the colony. Their function is to collect honey, look after the young ones, clean the comb, defend the hive and maintain the temperature of the bee hive.
Question 3.
Explain the methods used for vermicomposting.
Answer:
Vermicomposting methods can range from a wormbin in the kitchen for household scraps to large mechanized systems, which can be able to accommodate tons of organic material. In general these methods are of the following types:
- Bin (or) Container method
- Vermicomposting of organic wastes in field pits
- Vermicomposting of organic wastes on ground heaps
Bin method:
Vermicomposting by bin method is the rearing of earthworms in a container or bin. The container is half filled with bedding materials such as shredded cardboard, leaves, paddy husk, chopped straw, saw dust and manure. Small quantity of soil and sand is added to provide necessary grit for the worms. The bedding material should be moistened by adding water that enables free movements of the worms. The worms are gently placed and spread evenly on the bedding.
Organic wastes (kitchen wastes, vegetable and fruit wastes) are added which are fed by the earthworms. The bin is covered with coconut leaves or gunny bags to conserve moisture, provide darkness and keep out of pests. After a period of 60 days the wastes are completely transformed into nutrient-rich materials that are excreted by earthworms known as worm castings. These castings are harvested and used as organic manure.
Question 4.
What are concentrates? Why should they be given to cattle?
Answer:
Concentrates are low in fibre and contain high level of carbohydrates, protein and other nutrients. A variety of raw materials such as cholam (jowar), kambu (pearl millet), ragi (finger millet), rice bran, wheat bran, cotton seed cake, mustard cake, linseed cake, groundnut cake, mango seed, neem cake and yellu (sesame) cake can be used to make concentrate feed. The concentrates are fed at the time of milking. This helps in ‘let down’ of milk.
The daily average feed ratio of a milking cow is:
- 15-25 kg of roughage (dry grass and green fodder)
- 4-5 kg of grain mixture
- 100-150 litres of water
For a cow that gives above 2.5 kg milk yield per day, 1 kg of concentrate feed should be given for every additional milk yield.