Tamilnadu Samacheer Kalvi 12th Chemistry Notes Chapter 12 Carbonyl Compounds Notes
Carbonyl compounds: An organic compounds, which contains 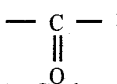 is called carbonyl compounds. They plays an important role in the metabolic process. Carbonyl compounds are important constituents of fabrics, plastics and drugs.
is called carbonyl compounds. They plays an important role in the metabolic process. Carbonyl compounds are important constituents of fabrics, plastics and drugs.
Ozonolysis: Alkenes or Alkynes reacts with ozone to form ozonide which on subsequent cleavage with zinc and water gives corresponding aldehydes or ketones. This process is known as ozonolysis.
Hydration of alkynes: The hydration of alkynes in the presence of 40% dilute sulphuric acid and 1% H2SO4 to give the corresponding aldehydes or ketones.
Rosenmund reduction: Aldehydes can be prepared by the hydrogenation of acid chloride, in the presence of palladium supported by barium sulphate. This reaction is called Rosenmund reduction.
Stephen’s reaction: When alkylcyanides are reduced using SnCl2 / HCl, imines are formed, which on hydrolysis gives corresponding aldehyde. This reaction is called Stephen’s reaction.
Selective reduction of cyanides: Diisobutyl aluminium hydride (DIBAL -H) selectively reduces the alkyl cyanide to form imines which on hydrolysis gives aldehydes.
Etard reaction: When chromylchloride is used as an oxidising agent, toluene gives benzaldehyde. This reaction is called Etard reaction.
Gattermann – Koch reaction: In this reaction, carbon monoxide and HC1 generate formyl cation intermediate, which attacks the aromatic ring to form corresponding aldehydes.
Urotropine: Formaldehyde reacts with ammonia to form hexa methylene tetramine, which is also known as Urotropine.
Carbonyl compounds: An organic compounds, which contains c is called carbonyl compounds. They plays an important role in the metabolic process. Carbonyl compounds are important constituents of fabrics, plastics and drugs.
Ozonolysis: Alkenes or Alkynes reacts with ozone to form ozonide which on subsequent cleavage with zinc and water gives corresponding aldehydes or ketones. This process is known as ozonolysis.
Hydration of alkynes: The hydration of alkynes in the presence of 40% dilute sulphuric acid and 1% H2SO4 to give the corresponding aldehydes or ketones.
Rosenmund reduction: Aldehydes can be prepared by the hydrogenation of acid chloride, in the presence of palladium supported by barium sulphate. This reaction is called Rosenmund reduction.
Stephen’s reaction: When alkylcyanides are reduced using SnCl2 / HCl, imines are formed, which on hydrolysis gives corresponding aldehyde. This reaction is called Stephen’s reaction.
Selective reduction of cyanides: Diisobutyl aluminium hydride (DIBAL -H) selectively reduces the alkyl cyanide to form imines which on hydrolysis gives aldehydes.
Etard reaction: When chromylchloride is used as an oxidising agent, toluene gives benzaldehyde. This reaction is called Etard reaction.
Gattermann – Koch reaction: In this reaction, carbon monoxide and HCl generate formyl cation intermediate, which attacks the aromatic ring to form corresponding aldehydes.
Urotropine: Formaldehyde reacts with ammonia to form hexa methylene tetramine, which is also known as Urotropine.
Uses of Urotropine:
- Urotropine is used as a medicine to treat urinary infection.
- Nitration of Urotropine under controlled condition gives an explosive RDX (Research and development explosive). It is also called cyclonite or cyclotri methylene trinitramine.
Popoff s rule: It states that during the oxidation of an unsymmetrical ketone, a (C-CO) bond is cleaved in such a way that the keto group stays with the smaller alkyl group.
Clemmensen reduction: Aldehydes and Ketones when heated with zinc amalgam and concentrated hydrochloric acid gives hydrocarbons. This reduction is called Clemmensen reduction.
Wolf – Kishner reduction: Aldehydes and Ketones when heated with hydrazine and sodium ethoxide, hydrocarbons are formed. This reduction is called Wolf – Kishner reduction.
Aldol condensation: In the presence of bases like NaOH, or KOH, two molecules of an aldehyde or ketone having α – hydrogen add together to give (β- hydroxyl aldehyde (aldol) or β – hydroxyl ketone (ketol). This reaction is called aldol condensation reaction.
Crossed aldol condensation: Aldol condensation can also take place between two different aldehydes or ketones or between one aldehyde and one ketone such an aldol condensation is called crossed aldol condensation.
Claisen – Schmidt Condensation: Benzaldehyde condenses with aliphatic or methyl ketone in the presence of dil. alkali at room temperature to form unsaturated aldehyde or ketone. This type of reaction is called Claisen – Schmidt condensation.
Cannizaro reaction: In the presence of concentrated aqueous alkali, aldehydes which do not have a – hydrogen atom under of self oxidation and reduction to give a mixture of alcohol and a salt of carboxylic acid. This reaction is called Cannizaro reaction.
Crossed Cannizaro reaction: When Cannizaro reaction takes place between two different aldehydes (neither containing an a – hydrogen atom), the reaction is called as cross cannizaro reaction.
Benzoin condensation: This reaction involves the treatment of an aromatic aldehyde with alcoholic KCN. The products are hydroxyl ketone.
Perkin’s reaction: When an aromatic aldehyde is heated with an aliphatic acid anhydride in the presence of the sodium salt of the acid corresponding to the anhydride, condensation takes place and an a, p unsaturated acid is obtained. This reaction is known as Perkin’s reaction.
Knoevenagal reaction: Benzaldehyde condenses with malonic acid in the presence of pyridine forming cinnamic acid is called Knoevenagal reaction or condensation.
Schiff’s base: Aromatic aldehydes react with primary amines in the presence of an acid to form schiff’s base.
Tollens Reagent Test: Tollens reagent is an ammonical silver nitrate solution. When an aldehyde is warmed with Tollens reagent a bright silver mirror is produced due to the formation of silver metal. This reaction is also called silver mirror test for aldehydes.
Fehlings solution Test: Fehlings solution is prepared by mixing equal volumes of Fehlings solution ‘A’ containing aqueous copper sulphate and Fehlings solution ‘B’ containing alkaline solution of sodium potassium tartarate (Rochelle salt). When aldehyde is warmed with Fehlings solution deep blue colour solution is changed to red precipitate of cuprous oxide.
Benedict’s solution Test: Benedicts solution is a mixture of CuSO4 + sodium citrate + NaOH. Cu2+ is reduced by aldehyde to give red precipitate of cuprous oxide.
Schiffs’ reagent Test: Dilute solution of aldehydes when added to schiffs’ reagent (Rosaniline hydrochloride dissolved in water and its red colour decolourised by passing S02) yields its red colour. This is known as Schiffs’ test for aldehydes. Ketones do not give this test. Acetone however gives a positive test but slowly.
Formalin: 40% aqueous solution of formaldehyde is called formalin. It is used for preserving biological specimens
Uses of Acetaldehyde:
- Acetaldehyde is used for silvering of mirrors
- Paraldehyde is used in medicine as a hypnotic
- Acetaldehyde is used in the commercial preparation of number of organic compounds like ‘ acetic acid, ethyl acetate etc.,
Uses of Acetone:
- Acetone is used as a solvent, in the manufacture of smokeless powder (cordite).
- It is used as a nail polish remover.
- It is used in the preparation of sulphonal, a hypnotic.
- It is used in the manufacture of thermosoftening plastic Perspex.
Uses of Benzaldehyde:
- as a flavoring agent
- in perfumes
- in dye intermediates
- as starting material for the synthesis of several other organic compounds like cinnamaldehyde, cinnamic acid, benzoyl chloride etc.
Uses of Aromatic Ketones:
- Acetophenone has been used in perfumery and as a hypnotic under the name hyphone.
- Benzophenone is used in perfumery and in the preparation of benzhydrol drop.
Carboxylic acids: An organic compounds containing a carboxylic functional group, – COOH are called carboxylic acids. The Carboxyl group is the combination of carbonyl group and the hydroxyl group.
Vinegar: It is a 6% to 8% solution of acetic acid in water.
Glacial acetic acid: Pure acetic acid is called glacial acetic acid. Because it forms ice like crystal when cooled. When aqueous acetic acid is cooled at 289.5 K, it solidifies and forms ice like crystals, where as water remains in liquid state and removed by filtration. This process is repeated to obtain glacial acetic acid.
Esterification: When carboxylic acids are heated with alcohols in the presence of cone. H2SO4 or dry HCl gas esters are formed. The reaction is reversible and is called esterification.
Decarboxylation: Removal of CO2 from carboxyl group is called as decarboxylation. Carboxylic acids lose carbon-di-oxide to form hydro carbon when their sodium salts are heated with soda lime (NaOH and CaO in the ratio 3:1)
Kolbe’s electrolytic reaction: The aqueous solutions of sodium or potassium salts of carboxylic acid on electrolysis gives alkanes at anode. This reaction is called kolbes electrolysis.
Hell – Volhard – Zelinsky reaction: Carboxylic acids having an a – hydrogen are
halogenated at the α – position on treatment with Cl2 or Br2 in the presence of small amount of red phosphorus to form α – halo carboxylic acids. This reaction is known as Hell – Volhard – Zelinsky reaction (HVZ reaction)
Tests for carboxylic acid group:
- In aqueous solution carboxylic acid turn blue litmus red.
- Carboxylic acids give brisk effervescence with sodium bicarbonate due to the evolution of carbon-di -oxide.
- When carboxylic acid is warmed with alcohol and Con H2SO4 it forms an ester, which is detected by its fruity odour.
Functional derivatives of carboxylic acids: Compounds such as acid chlorides,amides,esters etc., are called carboxylic acid derivatives because they differ from a carboxylic acid only in the nature of the group or atom that has replaced the – OH group of carboxylic acid.
Relative reactivity of Acid derivatives: The reactivity of the acid derivatives follows the
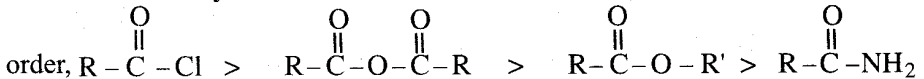
Order of reactivity of the acid derivatives with nucleophilic reagent follows the order:
Acid halide > acid anhydride > esters > acid amides
Transesterification: Ester of an alcohol can react with another alcohol in the presence of a mineral acid to give the ester of second alcohol. The interchange of alcohol portions of the esters is termed transesterification.
Ammonolysis: Esters react slowly with ammonia to form amides and alcohol. This reaction is called ammonolysis.
Claisen Condensation: Esters containing at least one a – hydrogen atom undergo self condensation in the presence of a strong base such as sodium ethoxide to form P – keto ester
Hoff mann’s degradation reaction: Amides reacts with bromine in the presence of caustic potash (KOH) to form a primary amine carrying one carbon less than the parent amide. This is called Hoff mann’s degradation reaction.
Uses of Formic acid:
- For the dehydration of hides
- As a coagulating agent for rubber latex
- In medicine for treatment of gout
- As an antiseptic in the preservation of fruit juice
Uses of Acetic acid:
- As table vinegar
- For coagulating rubber latex
- For manufacture of cellulose acetate and poly vinylacetate
Uses of Benzoic acid:
- As food preservative either in the pure form or in the form of sodium benzoate
- In medicine as an urinary antiseptic
- For manufacture of dyes
Uses of Acetyl chloride:
- As acetylating agent in organic synthesis
- In detection and estimation of – OH, – NH2 groups in organic compounds
Uses of Acetic anhydride:
- Acetylating agent
- In the preparation of medicine like asprin and phenacetin
- For the manufacture plastics like cellulose acetate and poly vinyl acetate.
Uses of Ethyl acetate:
- In the preparation of artificial fruit essences
- As a solvent for lacquers.
- In the preparation of organic synthetic reagent like ethyl acetoacetate.
Uses of Acetamide: Acetamide is used in the preparation of Primary amines.
Flavours of some of the esters are given below:
| Ester | Flavour |
| 1. Amyl acetate | Banana |
| 2. Ethyl butyrate | Pineapple |
| 3. Octyl acetate | Orange |
| 4. Isobutyl formate | Raspberry |
| 5. Amyl butyrate | Apricot |