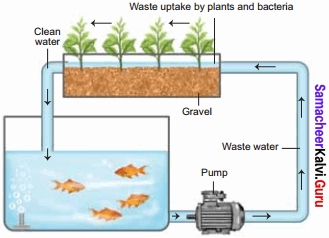
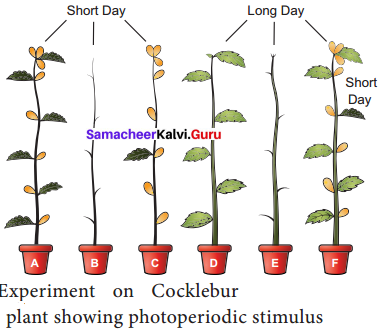
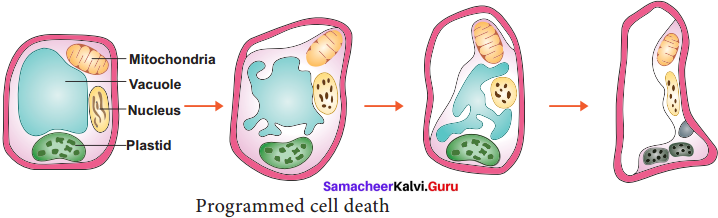
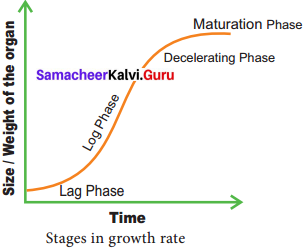
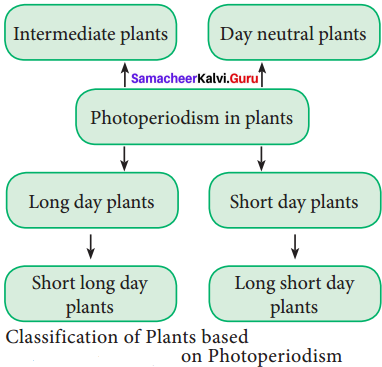
Students who are in search of 11th Bio Botany exam can download this Tamilnadu State Board Solutions for 11th Bio Botany Chapter 4 Reproductive Morphology from here for free of cost. These cover all Chapter 4 Reproductive Morphology Questions and Answers, PDF, Notes, Summary. Download the Samacheer Kalvi 11th Biology Book Solutions Questions and Answers by accessing the links provided here and ace up your preparation.
Tamilnadu Samacheer Kalvi 11th Bio Botany Solutions Chapter 4 Reproductive Morphology
Kickstart your preparation by using this Tamilnadu State Board Solutions for 11th Bio Botany Chapter 4 Reproductive Morphology Questions and Answers get the max score in the exams. You can cover all the topics of Chapter 4 Reproductive Morphology Questions and Answers easily after studying the Tamilnadu State Board 11th Bio Botany Textbook solutions pdf.
Samacheer Kalvi 11th Bio Botany Reproductive Morphology Text Book Back Questions and Answers
Choose the correct answer
Question 1.
Vexillary aestivation is characteristic of the family …………… .
(a) Fabaceae
(b) Asteraceae
(c) Solanaceae
(d) Brassicaceae
Answer:
(a) Fabaceae
Question 2.
Gynoecium with united carples is termed as …………… .
(a) apocarpous
(b) multicarpellary
(c) syncarpous
(d) none of the above
Answer:
(c) syncarpous
Question 3.
Aggregate fruit develops from …………… .
(a) multicarpellary, apocarpous ovary
(b) multicarpellary, syncarpous ovary
(c) multicarpellary ovary
(d) whole inflorescence
Answer:
(a) multicarpellary, apocarpous ovary
Question 4.
In an inflorescence where flowers are borne laterally in an acropetal succession the position of the youngest floral bud shall be …………… .
(a) proximal
(b) distal
(c) intercalary
(d) anywhere
Answer:
(b) distal
Question 5.
A true fruit is the one where …………… .
(a) only ovary of the flower develops into fruit
(b) ovary and calyx of the flower develops into fruit
(c) ovary, calyx and thalamus of the flower develops into fruit
(d) all floral whorls of the flower develops into fruit
Answer:
(a) only ovary of the flower develops into fruit
Question 6.
Find out the floral formula for a bisexual flower with bract, regular, pentamerous, distinct calyx and corolla, superior ovary without bracteole?
Answer:
![]()
Question 7.
Give the technical terms for the following:
(a) A sterile stamen
(b) Stamens are united in one bunch
(c) Stamens are attached to the petals
Answer:
(a) A sterile stamen – Staminode
(b) Stamens are united in one bunch – Monadelphous
(c) Stamens are attached to the petals – Epipetalous (petalostemonous)
Question 8.
Explain the different types of placentation with example.
Answer:
The different types of placentation with example:
- Marginal: It is with the placentae along the margin of a unicarpellate ovary. Example: Fabaceae.
- Axile: The placentae arises from the column in a compound ovary with septa. Example: Hibiscus, tomato and lemon.
- Superficial: Ovules arise from the surface of the septa. Example: Nymphaeceae.
- Parietal: It is the placentae on the ovary walls or upon intruding partitions of a unilocular, compound ovary. Example: Mustard, argemone and cucumber.
- Free – central: It is with the placentae along the column in a compound ovary without septa. Example: Caryophyllaceae, Dianthus and primrose.
- Basal: It is the placenta at the base of the ovary. Example: Sunflower (Asteraceae) Marigold.
Question 9.
Differentiate between aggregate fruit with multiple fruit.
Answer:
1. Aggregate fruit:
Aggregate fruits develop from a single flower having an apocarpous pistil. Each of the free carpel is developed into a simple fruitlet. A collection of simple fruitlets makes an aggregate fruit. An individual ovary develops into a drupe, achene, follicle or berry. An aggregate of these fruits borne by a single flower is known as an etaerio. Example: Magnolia, Raspberry, Annona and Polyalthia.
2. Multiple or Composite fruit: A multiple or composite fruit develops from the whole inflorescence along with its peduncle on which they are borne.
- Sorosis: A fleshy multiple fruit which develops from a spike or spadix. The flowers fused together by their succulent perianth and at the same time the axis bearing them become fleshy or juicy and the whole inflorescence forms a compact mass. Example: Pineapple, Jack fruit and Mulberry.
- Syconus: A multiple fruit which develops from hypanthodium inflorescence. The receptacle develops further and converts into fleshy fruit which encloses a number of true fruit or achenes which develops from female flower of hypanthodium inflorescence. Example: Ficus.
Question 10.
Explain the different types of fleshy fruit with suitable example?
Answer:
The fleshy fruits are derived from single pistil, where the pericarp is fleshy, succulent and differentiated into epicarp, mesocarp and endocarp. It is subdivided into the following:
- Berry: Fruit develops from bicarpellary or multicarpellary, syncarpous ovary. Here the epicarp is thin, the mesocarp and endocarp remain undifferentiated. They form a pulp in which the seeds are embedded. Example: Tomato, date palm, grapes and brinjal.
- Drupe: Fruit develops from monocarpellary, superior ovary. It is usually one seeded. Pericarp is differentiated into outer skinny epicarp, fleshy and pulpy mesocarp and hard and stony endocarp around the seed. Example: Mango and coconut.
- Pepo: Fruit develops from tricarpellary inferior ovary. Pericarp terns leathery or woody which encloses, fleshy mesocarp and smooth endocarp. Example: Cucumber, watermelon, bottle gourd and pumpkin.
- Hesperidium: Fruit develops from multicarpellary, multilocular, syncarpous, superior ovary. The fruit wall is differentiated into leathery epicarp with oil glands, a middle fibrous mesocarp. The endocarp forms distinct chambers, containing juicy hairs. Example: Orange and lemon.
- Pome: It develops from multicarpellary, syncarpous, inferior ovary. The receptacle also develops along with the ovary and becomes fleshy, enclosing the true fruit. In pome the epicarp is thin skin like and endocarp is cartilagenous. Example: Apple and pear.
- Balausta: A fleshy indehiscent fruit developing from multicarpellary, multilocular inferior ovary whose pericarp is tough and leathery. Seeds are attached irregularly with testa being the edible portion. Example: Pomegranate.
Textbook Activity Solved
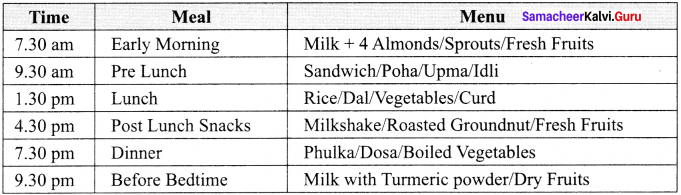
Prepare a diet chart to provide balanced diet to an adolescent (a school going child) which includes food items (fruits, vegetable and seeds) which are non – expensive and are commonly available.
Diet Chart for an Adolescent:
Samacheer Kalvi 11th Bio Botany Reproductive Morphology Additional Questions and Answers
I. Multiple Choice Questions
Choose the correct answer:
Question 1.
Placentation in tomato and lemon is …………… .
(a) parietal
(b) marginal
(c) free – central
(d) axile
Answer:
(d) axile
Question 2.
The coconut water and the edible part of coconut are equivalent to …………… .
(a) endosperm
(b) endocarp
(c) mesocarp
(d) embryo
Answer:
(a) endosperm
Question 3.
Geocarpic fruits are seen in …………… .
(a) carrot
(b) groundnut
(c) radish
(d) turnip
Answer:
(b) groundnut
Question 4.
Keel is characteristic petal of the flowers of …………… .
(a) Gulmohar
(b) Cassia
(c) Calotropis
(d) Bean
Answer:
(d) Bean
Question 5.
When the calyx is coloured and showy, it is called …………… .
(a) petaloid
(b) sepaloid
(c) bract
(d) spathe
Answer:
(a) petaloid
Question 6.
Bracts are modified leaves which bear flowers in their axils. Identify the plant which has a large showy brightly coloured bract …………… .
(a) Jasmine
(b) Euphorbia
(c) Hibiscus
(d) Bougainvillea
Answer:
(d) Bougainvillea
Question 7.
A flower which can be divided into equal vertical halves, by more than one plane of division is …………… .
(a) zygomorphic
(b) cyclic
(c) actinomorphic
(d) heteromorphic
Answer:
(c) actinomorphic
Question 8.
In Theobroma cocoa, the inflorescence arise from …………… .
(a) terminal shoot
(b) axillary part
(c) trunk of plant
(d) leaf node
Answer:
(c) trunk of plant
Question 9.
The type of inflorescence seen in Caesalpinia is …………… .
(a) corymb
(b) compound corymb
(c) capitulum
(d) umbel
Answer:
(a) corymb
Question 10.
Head is the characteristic of …………… family.
(a) Fabaceae
(b) Malvaceae
(c) Asteraceae
(d) Solanaceae
Answer:
(c) Asteraceae
Question 11.
Thyrsus is a type of …………… inflorescence.
(a) raceme
(b) cyme
(c) mixed
(d) special
Answer:
(c) mixed
Question 12.
Number of whorls in a complete flower is …………… .
(a) one
(b) two
(c) three
(d) four
Answer:
(d) four
Question 13.
Monoclinous flower will have …………… .
(a) androecium
(b) gynoecium
(c) both androecium & gynoecium
(d) none
Answer:
(c) both androecium & gynoecium
Question 14.
If unisexual and bisexual flowers are seen in same plant then the plant is said to be …………… .
(a) polyphyllous
(b) polygamous
(c) hermaphroditic
(d) dioecious
Answer:
(b) polygamous
Question 15.
…………… is a raceme of cymes.
(a) Verticil
(b) Cyathium
(c) Umbel
(d) Thyrsus
Answer:
(d) Thyrsus
Question 16.
Unit of perianth is …………… .
(a) petal
(b) sepal
(c) tepal
(d) stamen
Answer:
(c) tepal
Question 17.
Number of floral parts per whorl is called …………… .
(a) curosity
(b) atrocity
(c) merosity
(d) porosity
Answer:
(c) merosity
Question 18.
What is the green cap – like part of brinjal fruit?
(a) Corolla
(b) Perianth
(c) Calyx
(d) Pistil
Answer:
(c) Calyx
Question 19.
Butterfly shaped corolla is seen in …………… type.
(a) rosaceous
(b) caryophyllaceous
(c) cruciform
(d) papilionaceous
Answer:
(d) papilionaceous
Question 20.
Inflorescence seen in Daucas carota is …………… .
(a) umbel
(b) corymb
(c) compound umbel
(d) compound corymb
Answer:
(c) compound umbel
Question 21.
Arrangement of sepals and petals in flower bud is called …………… .
(a) adhesion
(b) aestivation
(c) placentation
(d) cohesion
Answer:
(b) aestivation
Question 22.
Which is not a part of pistil?
(a) Style
(b) Stigma
(c) Connective tissue
(d) carpel
Answer:
(c) Connective tissue
Question 23.
The type of calyx in brinjal is …………… .
(a) caducous
(b) deciduous
(c) persistent
(d) fugacious
Answer:
(c) persistent
Question 24.
Sterile stamen is called …………… .
(a) pistillode
(b) sessile
(c) staminode
(d) apostamen
Answer:
(c) staminode
Question 25.
Other name for gynoecium is …………… .
(a) carpel
(b) pistil
(c) style
(d) overy
Answer:
(b) pistil
Question 26.
Cavity found inside the ovary is called …………… .
(a) lobule
(b) locule
(c) lacuna
(d) labium
Answer:
(b) locule
Question 27.
Which part of saffron flower is used as flavouring agent?
(a) Carpel
(b) Anther
(c) Style
(d) Stigma
Answer:
(d) Stigma
Question 28.
If the ovary is inferior, then the flower is …………… .
(a) hypogynous
(b) epigynous
(c) perigynous
(d) epihypogynous
Answer:
(b) epigynous
Question 29.
Fabaceae members show …………… placentation.
(a) basal
(b) parietal
(c) superficial
(d) marginal
Answer:
(d) marginal
Question 30.
The side of the flower facing mother axis is called as …………… side.
(a) anterior
(b) posterior
(c) lateral
(d) dorsi – ventral
Answer:
(b) posterior
Question 31.
Which of the following option represents calyx?
(a) C
(b) Ca
(c) K
(d) Ka
Answer:
(c) K
Question 32.
…………… are the products of pollination & fertilization.
(a) Seeds
(b) Ovules
(c) Fruits
(d) Vegetables
Answer:
(c) Fruits
Question 33.
Study of fruits is called as …………… .
(a) honology
(b) pomology
(c) horticulture
(d) apology
Answer:
(b) pomology
Question 34.
Fruit wall can also be called as …………… .
(a) endocarp
(b) epicarp
(c) pericarp
(d) mericorp
Answer:
(c) pericarp
Question 35.
Which part of the apple fruit does we eat?
(a) Perianth
(b) Involucre
(c) Thalamus
(d) Bracteole
Answer:
(c) Thalamus
Question 36.
The false septum seen in siliqua fruits is …………… .
(a) frenulum
(b) micropyle
(c) raphae
(d) replum
Answer:
(d) replum
Question 37.
The type of fruit in Ricinus in …………… .
(a) lomentum
(b) cremocarp
(c) regma
(d) nut
Answer:
(c) regma
Question 38.
Jack fruit is an example for …………… .
(a) syconus
(b) siliqua
(c) sorosis
(d) nut
Answer:
(c) sorosis
Question 39.
Which of the following is not a schizocarpic fruit?
(a) Cremocarp
(b) Regma
(c) Samara
(d) Carcerulus
Answer:
(c) Samara
Question 40.
After fertilization …………… modifies into seed.
(a) ovary
(b) ovule
(c) carpel
(d) stigma
Answer:
(b) ovule
Question 41.
In groundnuts, which part nourishes the embryo?
(a) Endosperm
(b) Albumin
(c) Cotyledons
(d) Carpel
Answer:
(c) Cotyledons
Question 42.
…………… are the means for perpetuation of species.
(a) Fruits
(b) Seeds
(c) Corolla
(d) Flowers
Answer:
(b) Seeds
Question 43.
…………… is the second whorl of the flower.
(a) Calyx
(b) Corolla
(c) Gynoecium
(d) Perianth
Answer:
(b) Corolla
Question 44.
…………… is a ripened ovule.
(a) Carpel
(b) Pistil
(c) Seed
(d) Fruit
Answer:
(c) Seed
Question 45.
Imperfect flowers will have …………… essential whorl(s).
(a) only 1
(b) 2
(c) none
(d) 4
Answer:
(a) only 1
II. Very Short Answer Type Questions (2 Marks)
Question 1.
How will you define inflorescence?
Answer:
An inflorescence is a group of flowers arising from a branched or unbranched axis with a definite pattern.
Question 2.
Where does the inflorescence axis arise in cauliflorous type of inflorescence?
Answer:
In cauliflorous type, inflorescence developed directly from a woody trunk. Example: Theobroma cocoa.
Question 3.
Name any two mixed inflorescences.
Answer:
Two mixed inflorescences:
- Thyrsus and
- Verticillaster.
Question 4.
When a flower is said to be complete?
Answer:
A flower is said to be complete when it has all the four whorls (calyx, corolla, androecium & gynoecium).
Question 5.
What is a sessile flower?
Answer:
A flower without a pedicel or stalk is said to be sessile flower.
Question 6.
Define merosity.
Answer:
Number of floral parts per whorl is called merosity.
Question 7.
Write the units of (a) Perianth and (b) Calyx.
Answer:
The units of (a) Perianth and (b) Calyx:
- (a) Perianth – tepals and
- (b) Calyx – sepals
Question 8.
Name the three types of petals in papilionoceous corolla.
Answer:
The three types of petals in papilionoceous corolla:
- Vexillum (standard)
- wings (alae)
- petals (carina)
Question 9.
What is a staminode? Give example.
Answer:
Sterile stamen is called staminode. e.g. Cassia
Question 10.
Define Pollinium.
Answer:
When the pollen grains are fused together as a single main, it is said to be pollinium.
Question 11.
List out the parts of a pistil.
Answer:
Ovary, style and stigma.
Question 12.
Define aestivation.
Answer:
Arrangement of sepals and petals in a floral bud.
Question 13.
What is mother axis?
Answer:
The branch that bears the flower is called mother axis.
Question 14.
What do you understand by the term “Pomology”?
Answer:
The branch of horticulture that deals with the study of fruits and their cultivation is called pomology.
Question 15.
How the seeds are classified based on endosperm?
Answer:
(a) Albuminous seed or Endospermous seed.
(b) Ex – Albuminous seed or non – Endospermous seed.
Question 16.
What is Spathe?
Answer:
In spadix, entire inflorescence is covered by a brightly coloured or hard bract called a spathe.
Question 17.
Differentiate Apocarpous and Syncarpous ovary.
Answer:
Apocarpous and Syncarpous ovary:
- Apocarpous: A pistil contains two or more distinct carpels. Example: Annona
- Syncarpous: A pistil contains two or more carpels which are connate. Example: Citrus and Tomato
Question 19.
Give examples for following fruit types: (a) Berry (b) Hesperidium
Answer:
(a) Berry: Tomato
(b) Hesperidium: Orange
III. Short Answer Type Questions (3 Marks)
Question 1.
Distinguish between Monoecious & Dioecious.
Answer:
Between Monoecious & Dioecious:
- Monoecious: Both male and female flowers are present in the same plant, e.g., Coconut
- Dioecious: Male and female flowers are present on separate plants, e.g., Papaya
Question 2.
Explain Bilateral symmetry.
Answer:
In bilateral symmetry the flower can be divided into equal halves in only one plane. Zygomorphic flower can efficiently transfer pollen grains to visiting pollinators. Example: Pisum.
Question 3.
Differentiate Apopetalous from Sympetalous.
Answer:
Apopetalous from Sympetalous:
- Apopetalous (or) Polypetalous: Petals are distinct, e.g., Hibiscus.
- Sympetalous (or) Gamopetalous: Petals are fused, e.g., Datura.
Question 4.
Define Placentation & mention their types.
Answer:
The mode of distribution of placenta inside the ovary is called placentation. Types of placentation: Marginal, Axile, Superficial, Parietal, Free – central and Basal.
Question 5.
Write the floral formula for the Hibiscus rosasinensis.
Answer:
![]()
Question 6.
What are Parthenocarpic fruit?
Answer:
Development of fruits without fertilization are called Parthenocarpic fruit. They are seedless fruits. Example: Banana.
Question 7.
From which type of flowers does the aggregate fruit develops?
Answer:
Aggregate fruits develop from a single flower having an apocarpous pistil. Each of the free carpel is develops into a simple fruitlet. A collection of simple fruitlets makes an aggregate fruit.
Question 8.
Classify seeds based on their cotyledons.
Answer:
Based on the number of cotyledons present, two types of seeds are recognized.
- Dicotyledonous seed: Seed with two cotyledons.
- Monocotyledonous seed: Seed with one cotyledon.
Question 9.
List out any 3 significances of seed.
Answer:
3 significances of seed:
- The seed encloses and protects the embryo for next generation.
- Seeds of various plants are used as food, both for animals and human.
- Seeds are the products of sexual reproduction so they provide genetic variations and recombination in a plant.
Question 10.
What is the importance of inflorescence.
Answer:
Function of inflorescence is to display the flowers for effective pollination and facilitate seed dispersal. The grouping of flowers in one place gives a better attraction to the visiting pollinators and maximize the energy of the plant.
Question 11.
Draw the line diagram for the following inflorescence.
Answer:
(a) Simple Dichasium:

(b) Compound Dichasium:
IV. Long Answer Type Questions (5 Marks)
Question 1.
Explain the various types of Schizocarpic fruit.
Answer:
This fruit type of intermediate between dehiscent and indehiscent fruit. The fruit instead of dehiscing rather splits into number of segments, each containing one or more seeds. They are of following types:
- Cremocarp: Fruit develops from bicarpellary, syncarpous, inferior ovary and splitting into two one seeded segments known as mericarps. e.g., Coriander and Carrot.
- Carcerulus: Fruit develops from bicarpellary, syncarpous, superior ovary and splitting into four one seeded segments known as nutlets, e.g., Leucas, Ocimum and Abutilon.
- Lomentum: The fruit is derived from monocarpellary, unilocular ovary. A leguminous fruit, constricted between the seeds to form a number of one seeded compartments that separate at maturity, e.g., Desmodium, Arachis and Mimosa.
- Regma: They develop from tricarpellary, syncarpous, superior, trilocular ovary and splits into one – seeded cocci which remain attached to carpophore, e.g., Ricinus and Geranium.
Question 2.
Explain the different types of flowers based on the position of ovary.
Answer:
Based on the position of ovary, a flower can be classified as:
- Hypogynous: The term is used for sepals, petals and stamens attached at the base of a superior ovary, e.g. Malvaceae.
- Epihypogynous: The term is used for sepals, petals and stamens attached at the middle of the ovary (half – inferior), e.g. Fabaceae and Rosaceae.
- Epigynous: The term is used for sepals, petals and stamens attached at the tip of an inferior ovary, e.g. Cucumber, apple and Asteraceae.
- Perigynous: The term is used for a hypanthium attached at the base of a superior ovary.
- Epiperigynous: The term is used for hypanthium attached at the apex of an inferior ovary.
Question 3.
Classify the anthers based on their mode of attachment.
Answer:
The anthers based on their mode of attachment:
- Basifixed: (Innate) Base of anther is attached to the tip of filament, e.g., Brassica, Datura
- Dorsifixed: Apex of filament is attached to the dorsal side of the anther, e.g. Citrus, Hibiscus
- Versatile: Filament is attached to the anther at midpoint, e.g., Grasses
- Adnate: Filament is continued from the base to the apex of anther, e.g. Verbena, Ranunculus, Nelumbo.
Question 4.
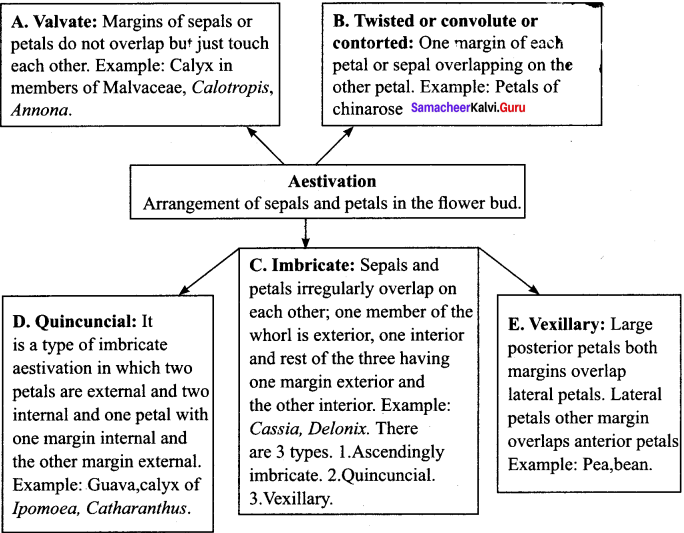
Define aestivation. Explain its types with example.
Answer:
Question 5.
Distinguish between racemose and cymose inflorescence.
Answer:
Racemose inflorescence
- Main axis of unlimited growth
- Flowers arranged in an acropetal succession
- Opening of flowers is centripetal
- Usually the oldest flower at the base of the inflorescence axis.
Cymose inflorescence:
- Main axis of limited growth
- Flowers arranged in a basipetal succession
- Opening of flowers is centrifugal
- Usually the oldest flower at the top of the inflorescence axis.
Question 6.
Write in detail about head inflorescence.
Answer:
Head: A head is a characteristic inflorescence of Asteraceae and is also found in some members of Rubiaceae. Example: Neolamarkia cadamba and Mitragyna parvifolia; and in some members of Fabaceae – Mimosoideae, example: Acacia nilotica, Albizia lebbeck, Mimosa pudica (sensitive plant). Torus contains two types of florets:
- Disc floret or tubular floret.
- Ray floret or ligulate floret.
Heads are classified into two types:
- Homogamous head: This type of inflorescence exhibits single kind of florets. Inflorescence has disc florets alone. Example: Vernonia, Ageratum or Ray florets alone. example: Launaea, Sonchus.
- Heterogamous head: The inflorescence possesses both types of florets. Example: Helianthus, Tridax.
Disc florets at the centre of the head are tubular and bisexual whereas the ray florets found at the margin of the head which are ligulate pistilate (unisexual).


Question 7.
List out the significance of fruits.
Answer:
The significance of fruits:
- Edible part of the fruit is a source of food, energy for animals.
- They are source of many chemicals like sugar, pectin, organic acids, vitamins and minerals.
- The fruit protects the seeds from unfavourable climatic conditions and animals.
- Both fleshy and dry fruits help in the dispersal of seeds to distant places.
- In certain cases, fruit may provide nutrition to the developing seedling.
- Fruits provide source of medicine to humans.
Question 8.
Draw a flow chart depicting the classification of fruits.
Answer:
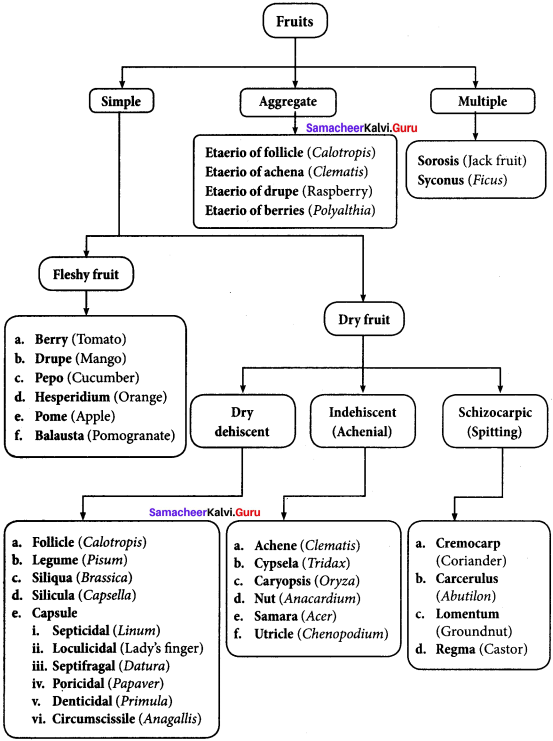
Question 9.
Explain in detail about any two special inflorescence.
Answer:
The inflorescences do not show any of the development pattern types are classified under special type of inflorescence.
1. Cyathium: Cyathium inflorescence consists of small unisexual flowers enclosed by a common involucre which mimics a single flower. Male flowers are organized in a scorpioid manner. Female flower is solitary and centrally located on a long pedicel. Male flower is represented only by stamens and female flower is represented only by pistil. Cyathium may be actinomorphic (Example: Euphorbia) or zygomorphic (Example: Pedilanthus) Nectar is present in involucre.
2. Hypanthodium: Receptacle is a hollow, globose structure consisting unisexual flowers present on the inner wall of the receptacle. Receptacle is closed except a small opening called ostiole which is covered by a series of bracts. Male flowers are present nearer to the ostiole, female and neutral flowers are found in a mixed manner from middle below. Example: Ficus sp. (Banyan and Pipal).
V. Higher Order Thinking Skills (HOTs)
Question 1.
Brinjal fruit has persistent calyx. Have you ever noticed the same in any other fruits? Name them.
Answer:
Tomato, Lady’s finger, guava and chilli also have persistent calyx.
Question 2.
Whether parthenocarpic fruits develop endosperm? Why?
Answer:
No, parthenocarpic fruits are developed without fertilization, but endosperm will form only after fertilization. So parthenocarpic fruits do not have endosperm.
Question 3.
Ovary develops into fruit after fertilization. While eating an Apple which part do you eat? Explain.
Answer:
Apple belongs to false fruit. In false fruits, apart from the ovary, non – carpellary parts also develop into fruit. In apple, the thalamus develops into fleshy edible part.
Question 4.
Cremocarp and Carcerulus both are schizocarpic fruits yet they differ. How?
Answer:
Cremocarp:
- Fruit develops from syncarpous inferior ovary.
- Ripened fruit split into two, one – seeded segments called mericarps.
Carcerulus:
- Fruit develops from syncarpous superior ovary.
- Ripened fruit split into four, one – seeded segments called nutlets.
Question 5.
Mango and coconut are ‘drupe’ type of fruits. In Mango, the edible part is fleshy mesocarp. What does the milk of tender coconut represent?
Answer:
Endosperm is the liquid (milk) potable part of tender coconut, which is rich in nutrients and is formed as a result of triple fusion.
Question 6.
Pick out correct ratio of the male flower to female flower in cyathium inflorescence and explain it?
(a) one : one
(b) one : many
(c) many : many and
(d) many : one.
Answer:
(a) One : one – Cyathium has single female flower represented by pistil and male flower represented by stamen.
Question 7.
Pollen differs from pollinium. How?
Answer:
Pollen are microspores which produces male gametes, whereas pollinium refers to the single mass of fused pollen grains.
Question 8.
Sunflower is not a flower – Justify your answer.
Answer:
Sunflower is actually an inflorescence not a single flower. The inflorescence of sunflower is capitulum composed of disc florets and ray florets.
Question 9.
Flower is a modified shoot for reproduction – Give possible evidence.
Answer:
Modified shoot for reproduction:
- Floral leaves (sepals and petals) are modified leaves.
- Floral and vegetative buds both emerge either in terminal or axillary position.
- Foliage leaves and floral leaves have identical arrangement on stem.
Question 10.
Is tomato a fruit or vegetable? Explain.
Answer:
Yes, tomato is a fruit. Because it develops from the ripened ovary and bears seeds, whereas vegetable refers to all other plant parts like root, stem and leaves.
Question 11.
What is caruncle? Where it is seen? How it helps the plant?
Answer:
Caruncle is the fleshy outgrowth at the base of seed. Usually caruncle helps in the seed dispersal, particularly by ants (Myrmecophily).
Question 12.
Both the prefixes (Uni – and Mono -) have the same meaning i.e. one in number. Does it mean that unisexual and monoecious are the same?
Answer:
That unisexual and monoecious:
- Unisexual refers to the sex of flower (i.e. whether it has anther or carpel).
- Monoecious refers to the plant bearing both the sexes in their flowers.
Believing that the Samacheer Kalvi 11th Biology Book Solutions Questions and Answers learning resource will definitely guide you at the time of preparation. For more details about Tamilnadu State 11th Bio Botany Chapter 4 Reproductive Morphology textbook solutions, ask us in the below comments and we’ll revert back to you asap.
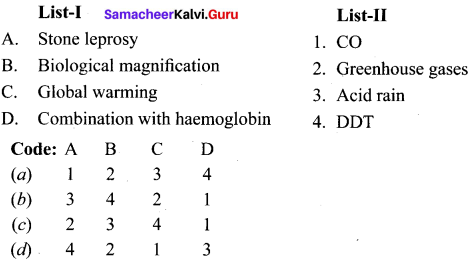
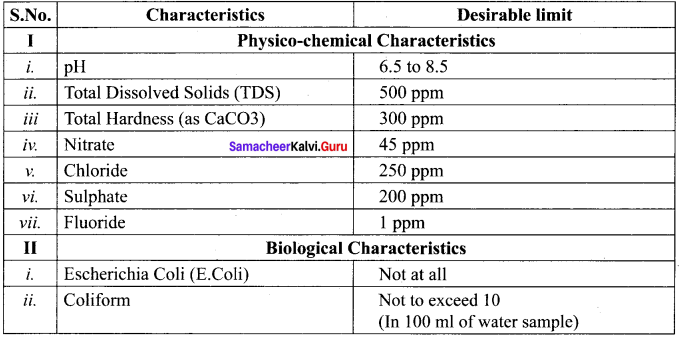
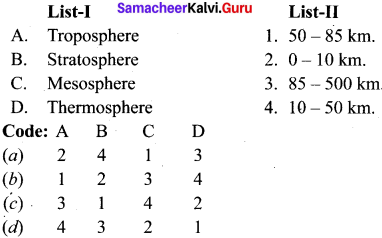
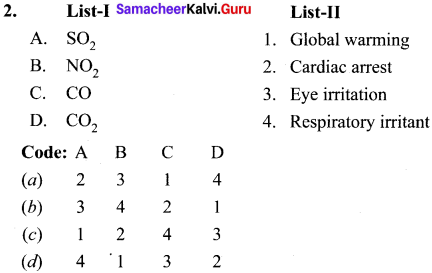


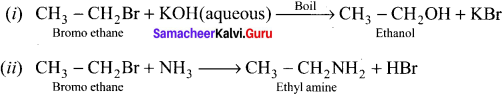
 is ………….
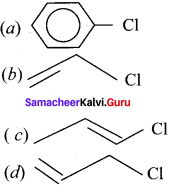
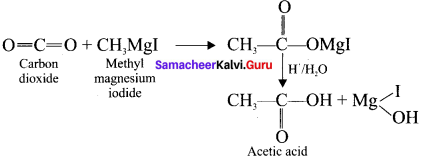
is …………. X + N2, X is …………
X + N2, X is …………

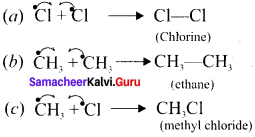
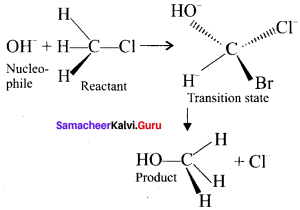
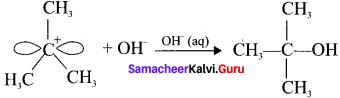
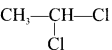
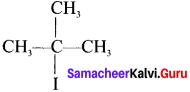
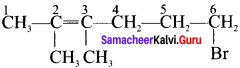
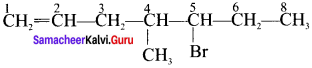
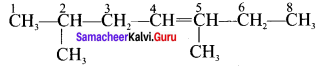
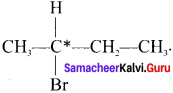 Here the C*is chiral carbon atom and it is surrounded by four different groups. Br
Here the C*is chiral carbon atom and it is surrounded by four different groups. Br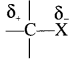
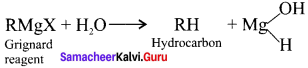
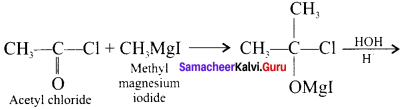
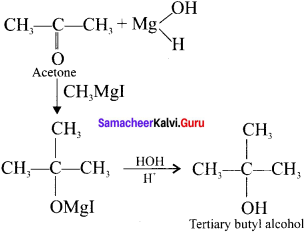
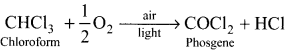
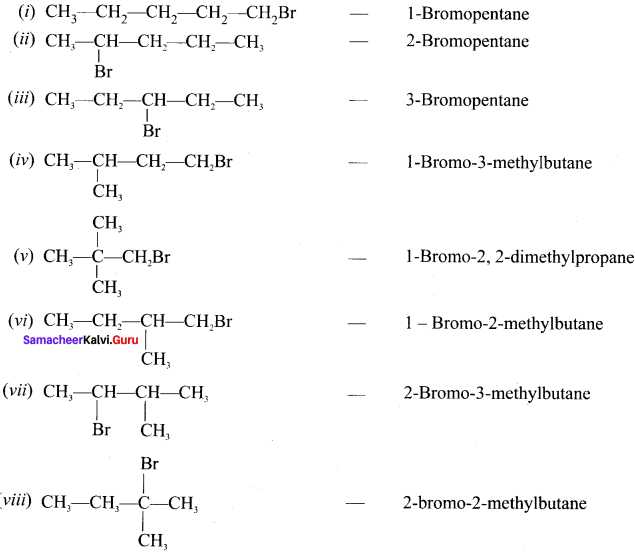
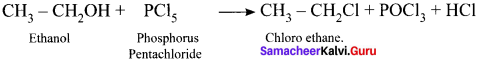
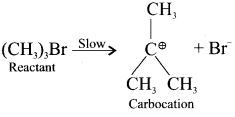
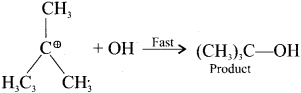
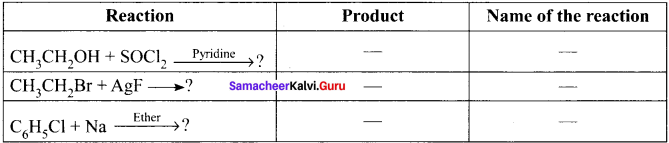
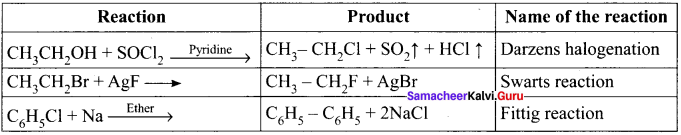
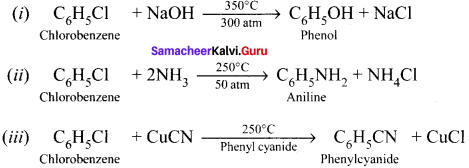


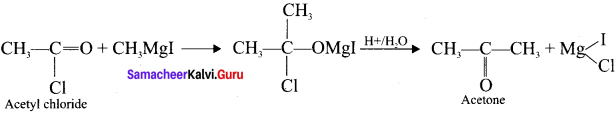


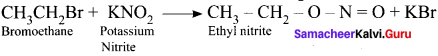
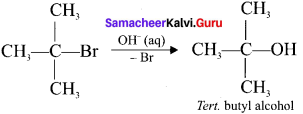
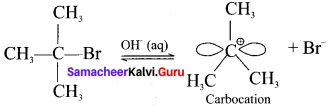
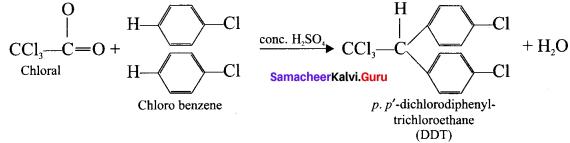
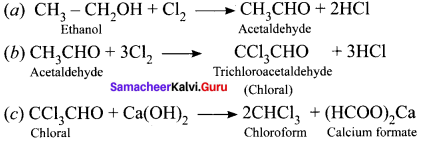
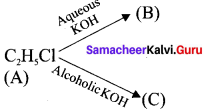
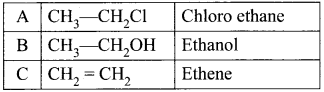
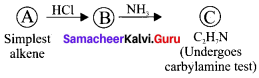
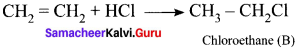
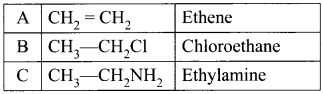
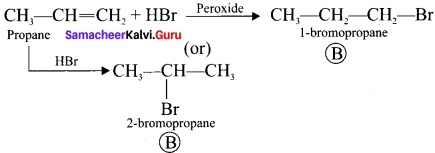
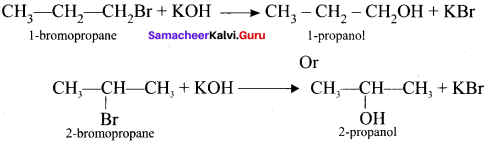
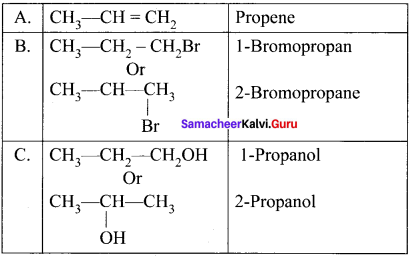
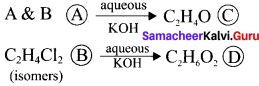
 and
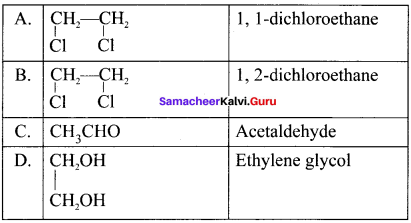
and 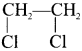 respectively with IUPAC names: 1, 1-dichloroethane (A) and 1.
respectively with IUPAC names: 1, 1-dichloroethane (A) and 1.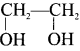 as (D).
as (D).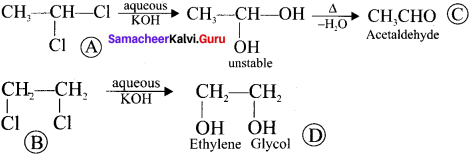
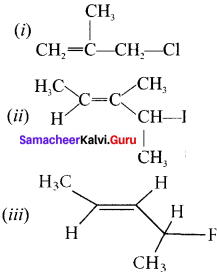
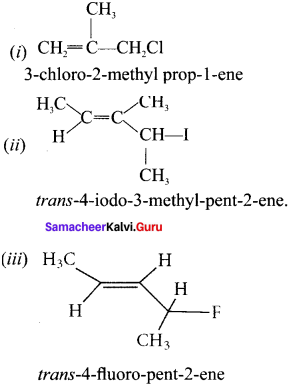
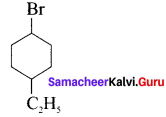
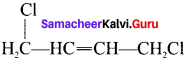
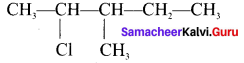
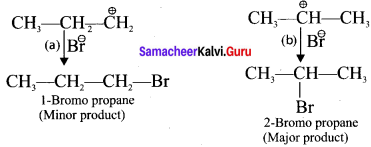
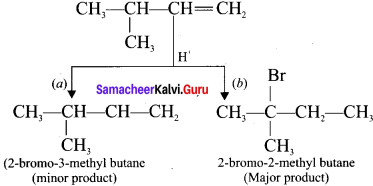
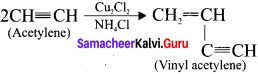
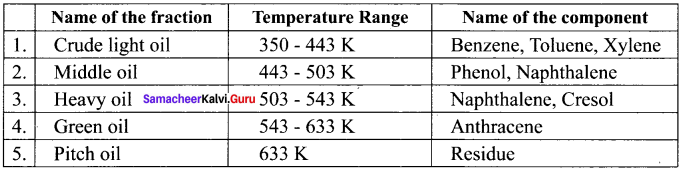
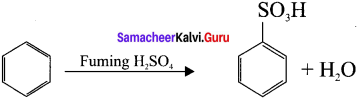
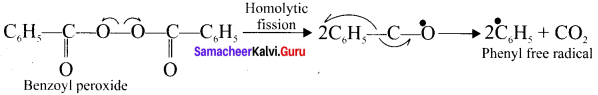
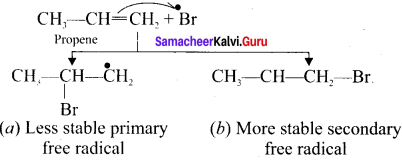
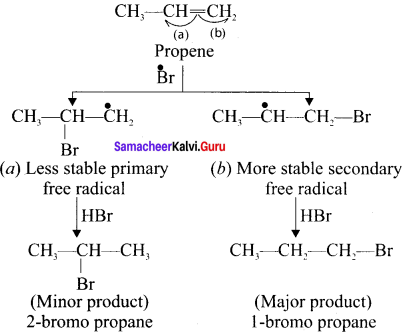
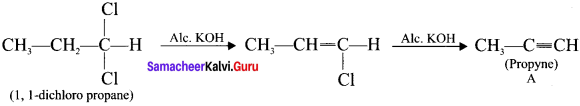
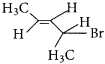
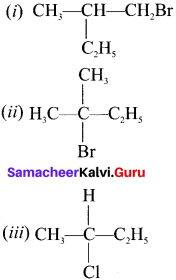
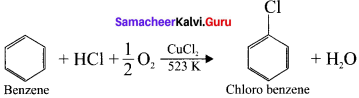
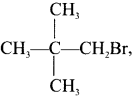
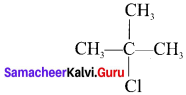
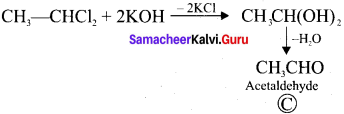
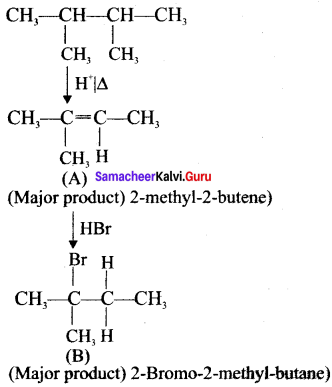
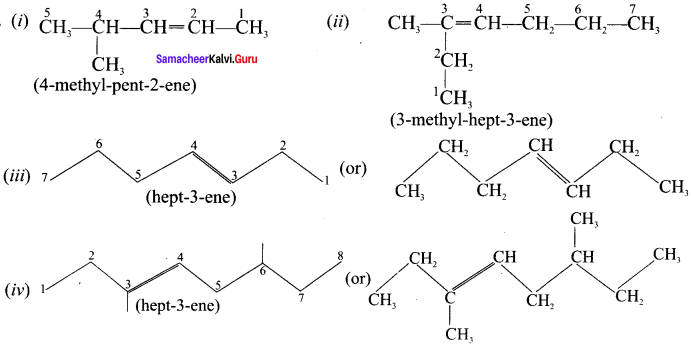
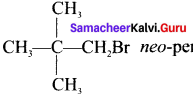 neo-pentyl bromide undergoes nucleophilic substitution reactions very slowly due to steric hindrance of many alkyl groups. When bromide is attached to neo-pentyl carbon atom, the heterolytic cleavage of C-Br takes place very slowly and substitution is also a very slow reaction.
neo-pentyl bromide undergoes nucleophilic substitution reactions very slowly due to steric hindrance of many alkyl groups. When bromide is attached to neo-pentyl carbon atom, the heterolytic cleavage of C-Br takes place very slowly and substitution is also a very slow reaction.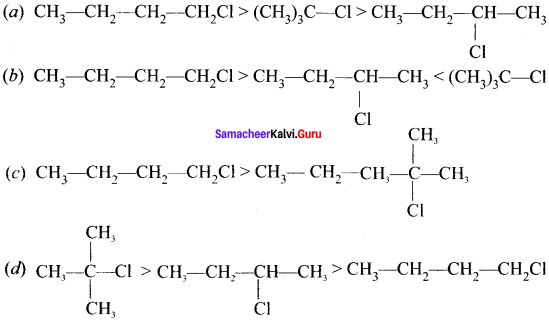
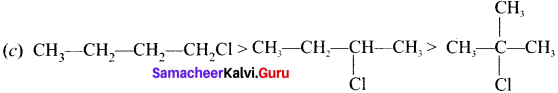
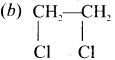
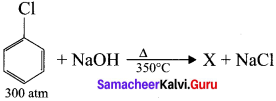
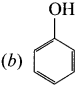
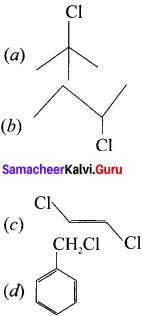
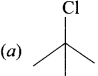
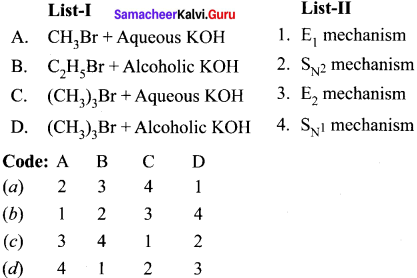
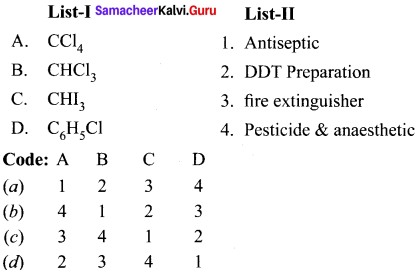
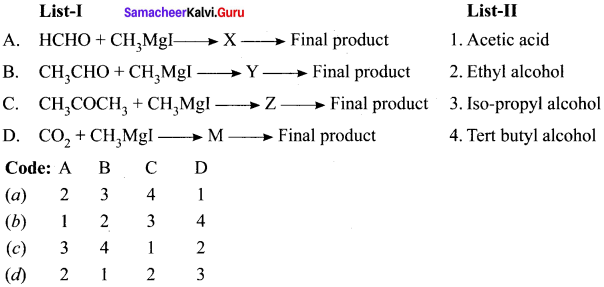
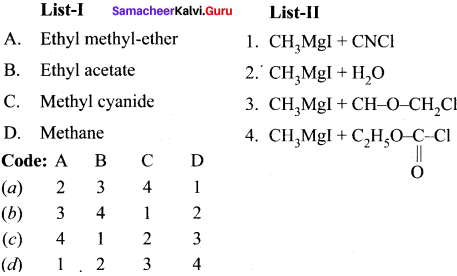

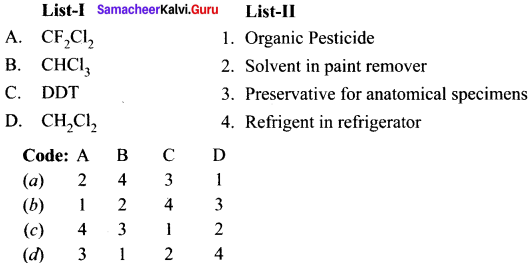
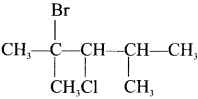 is ………
is ………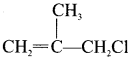 is ………
is ………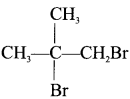 is ………..
is ………..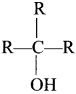
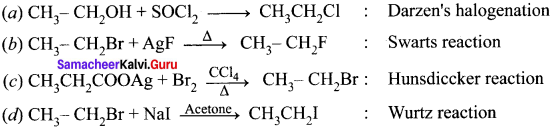
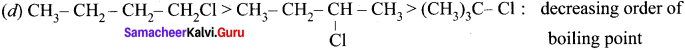
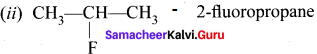
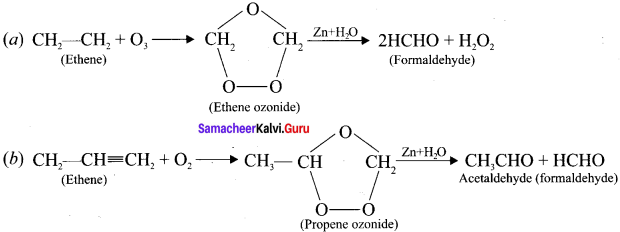
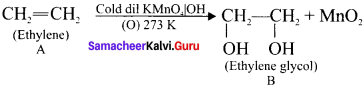
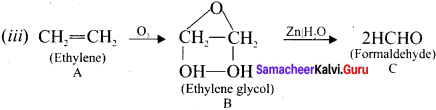
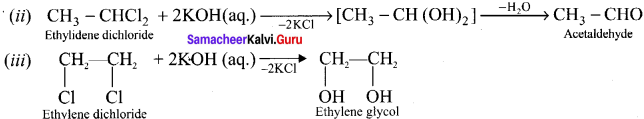
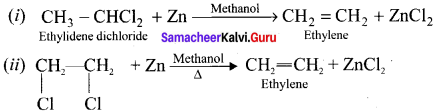
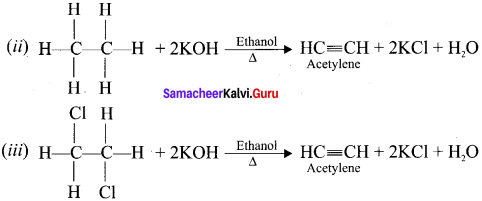
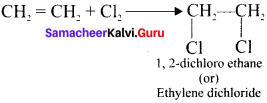
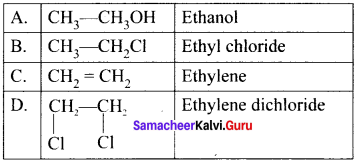
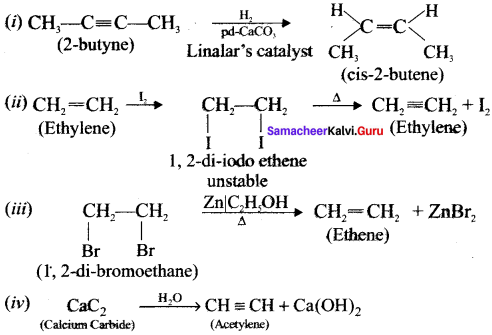
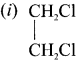 Ethylene dichioride (or) 1, 2-dichioroethane.
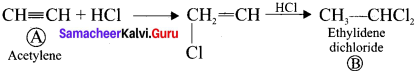
Ethylene dichioride (or) 1, 2-dichioroethane.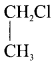 Ethylidene chloride (or) 2-dichioroethane.
Ethylidene chloride (or) 2-dichioroethane.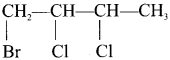

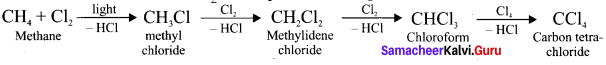
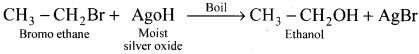
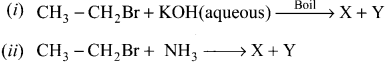
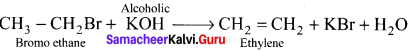

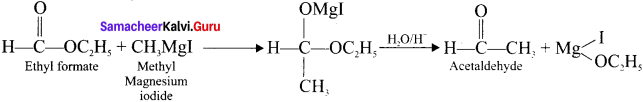
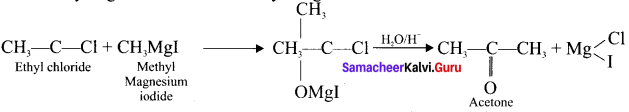
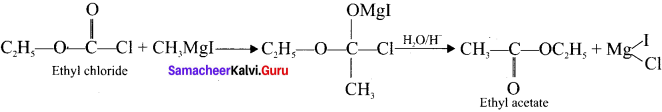

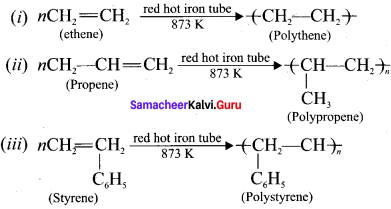
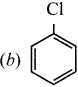
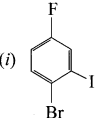 1 – bromo-4-fluoro-2-iodobenzene
1 – bromo-4-fluoro-2-iodobenzene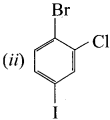 1 – bromo-2-fluoro-4-iodobenzene
1 – bromo-2-fluoro-4-iodobenzene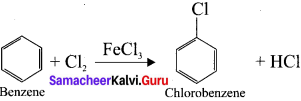
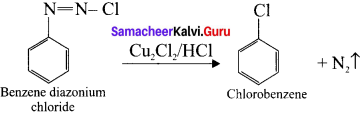
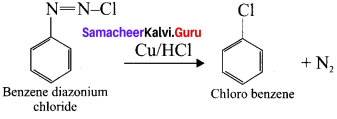
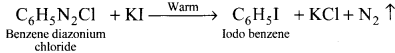
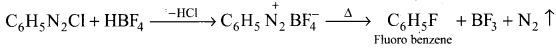


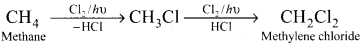
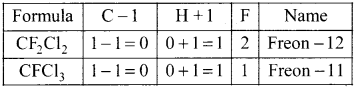
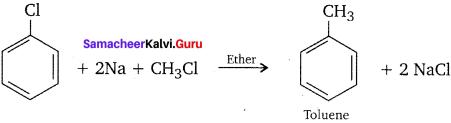
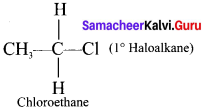
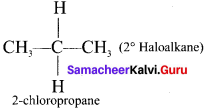
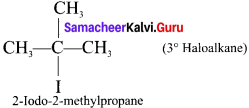
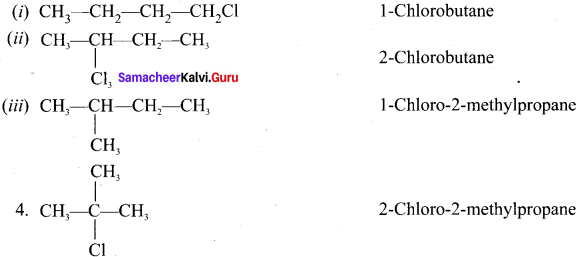
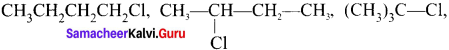
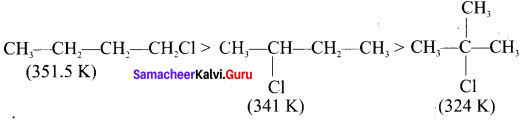
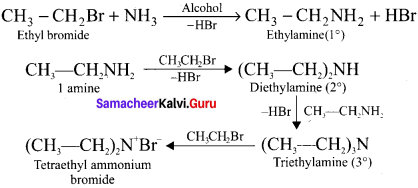

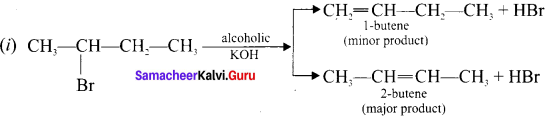
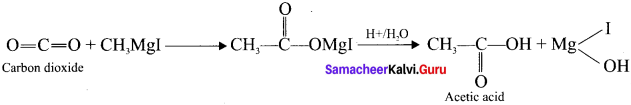
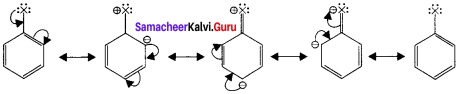
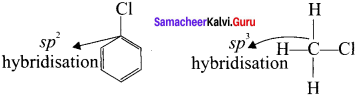
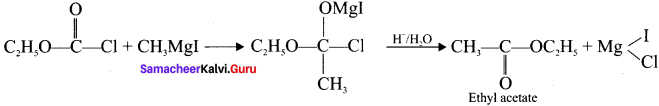
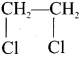 1,2-dichloroethane
1,2-dichloroethane

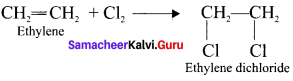
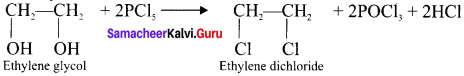
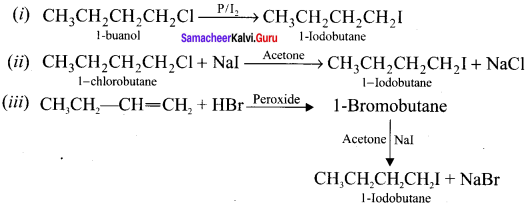
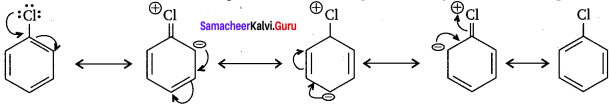
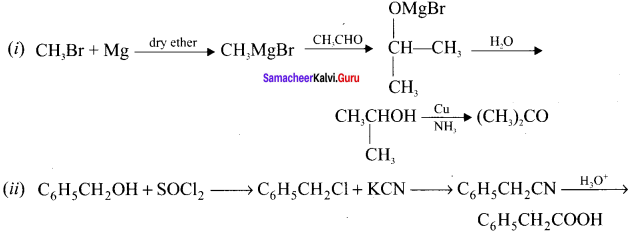
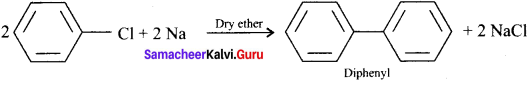
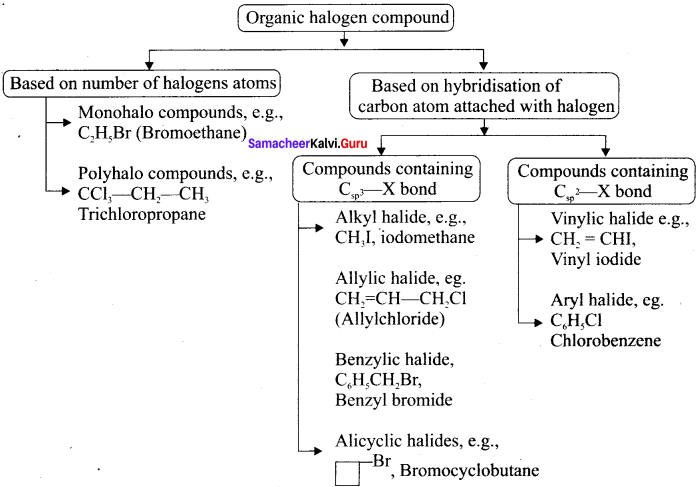
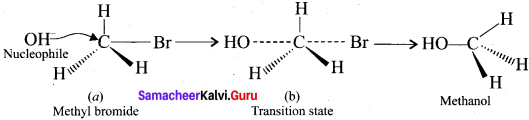
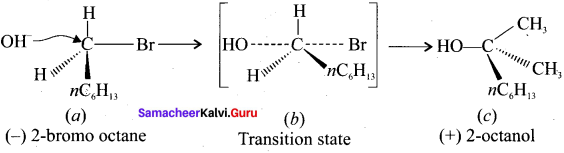

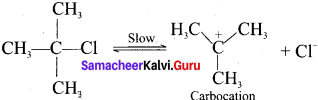
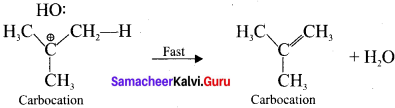
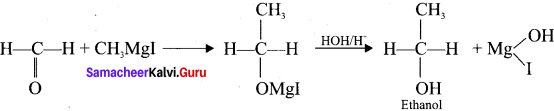
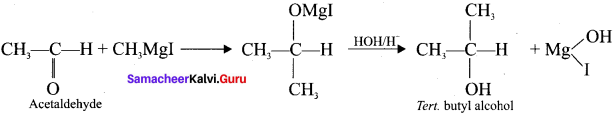
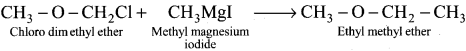
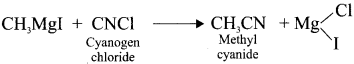

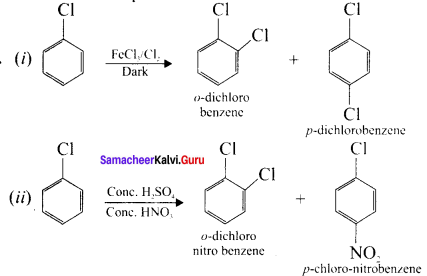
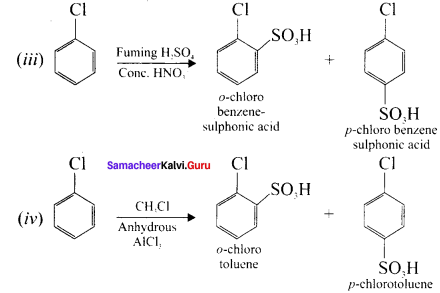


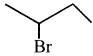
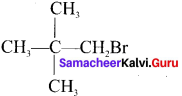
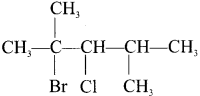
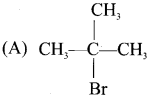 Tert butyl bromide and
Tert butyl bromide and 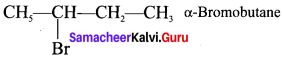
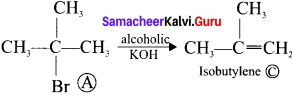
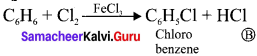
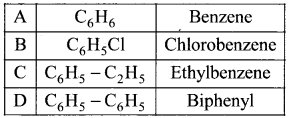
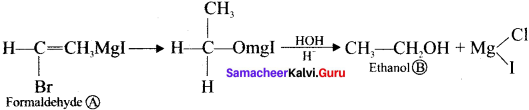
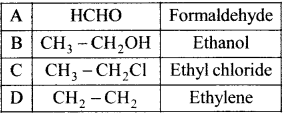
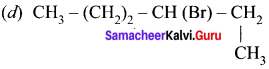
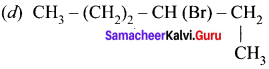
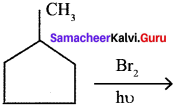

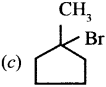
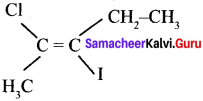
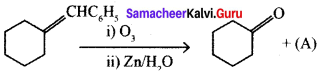
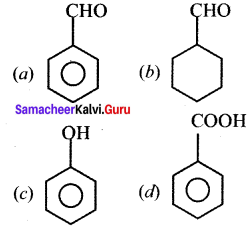
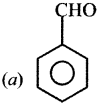
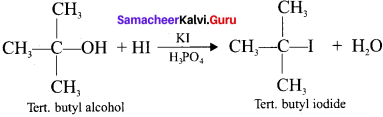
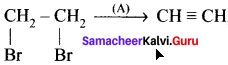 where a is ………
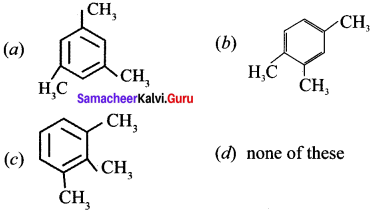
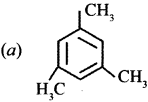
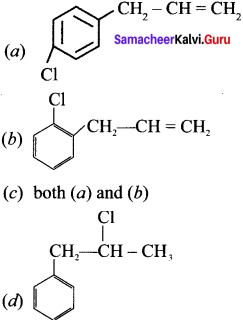
where a is ………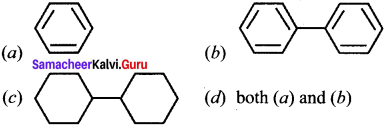

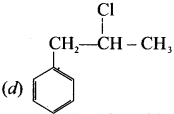
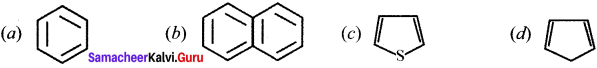
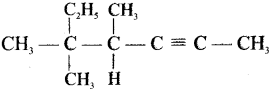
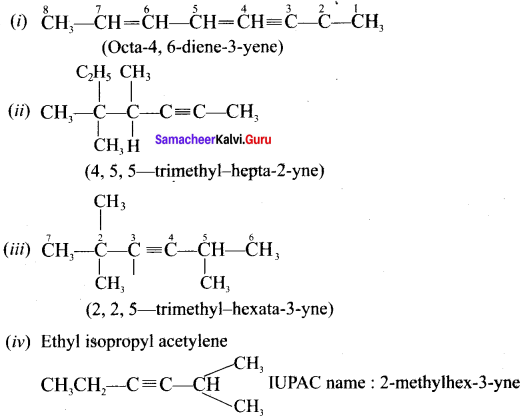
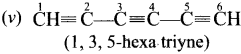
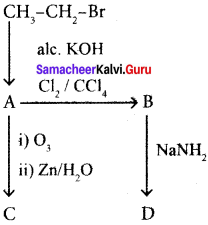
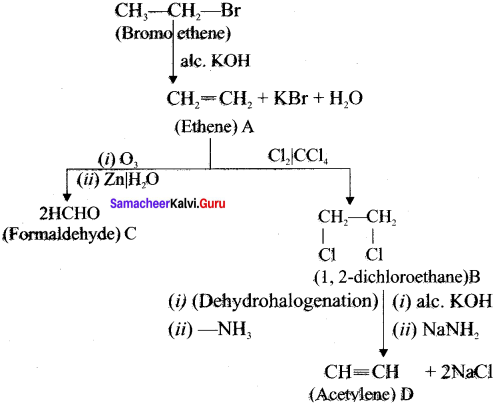
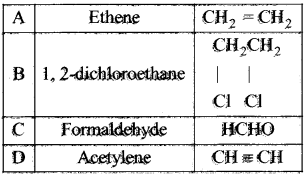
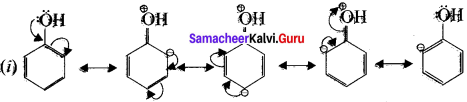
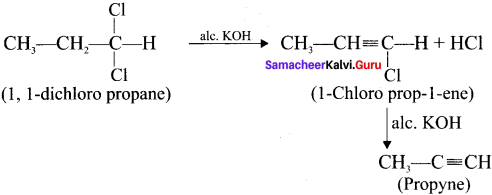
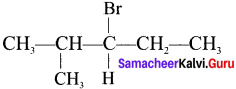
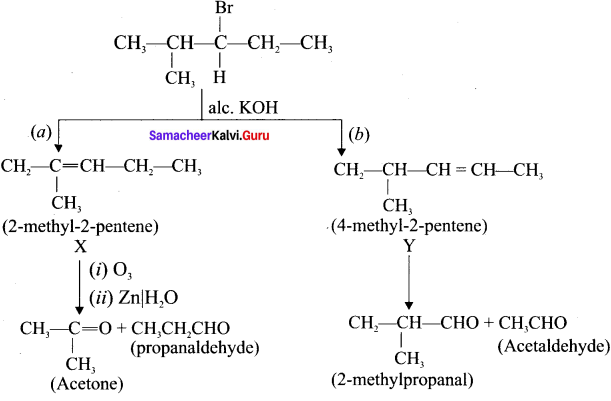
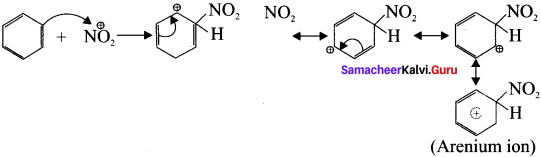
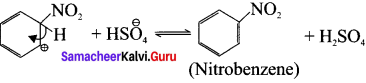

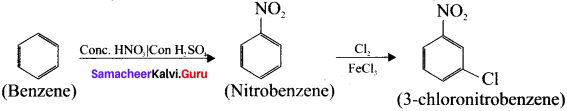
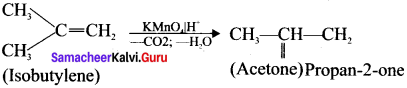
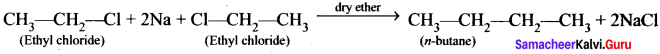
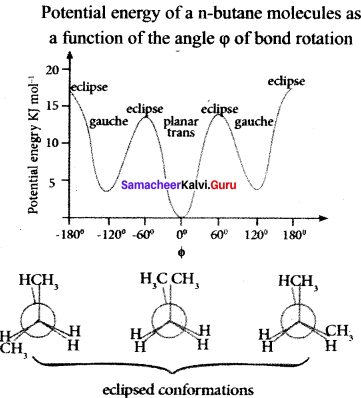
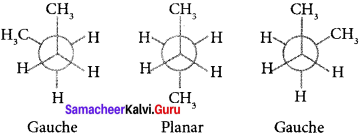
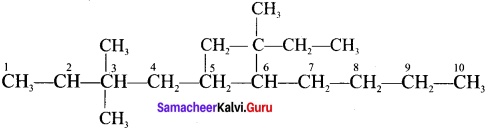
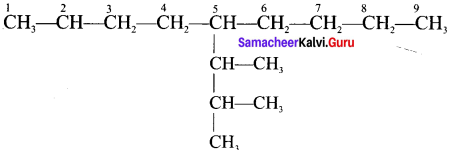
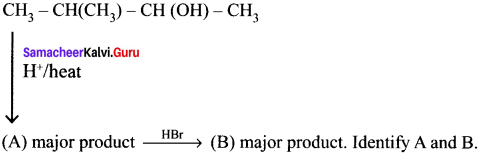
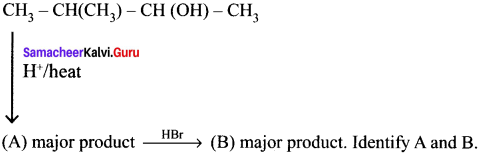

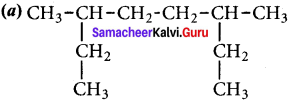
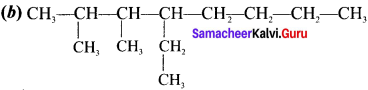
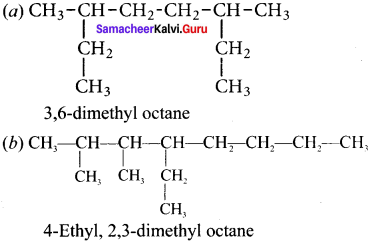
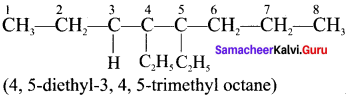
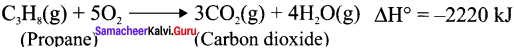

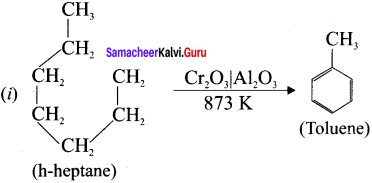
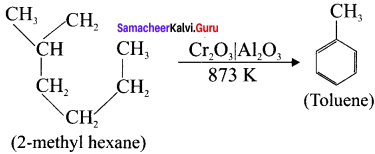
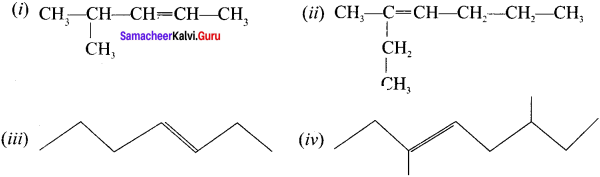
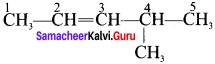

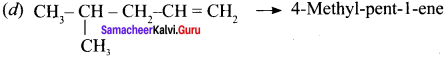
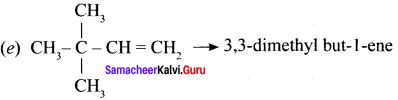
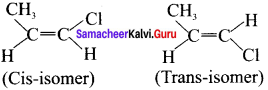


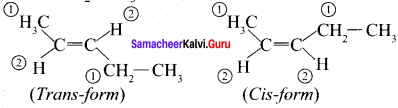
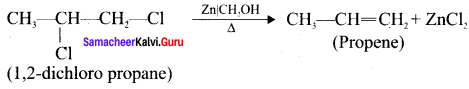
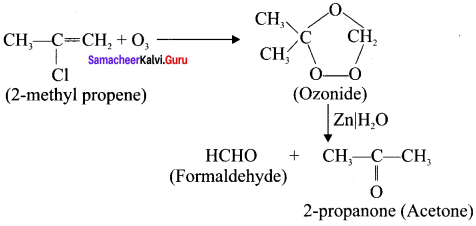
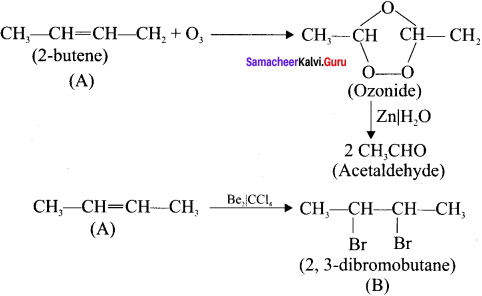

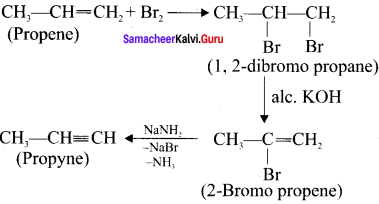
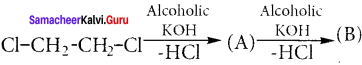
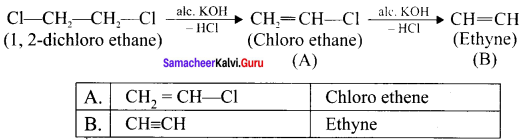
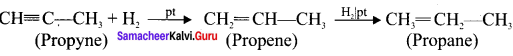


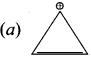
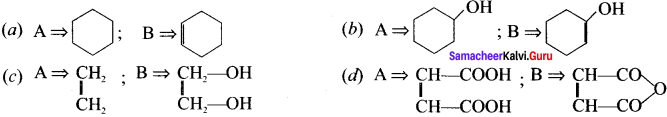
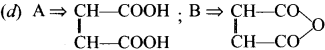





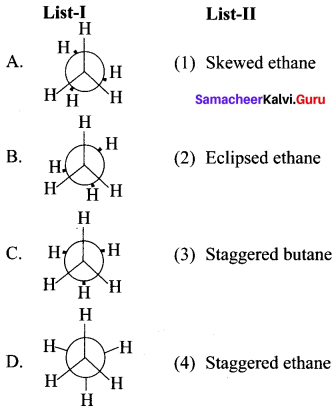





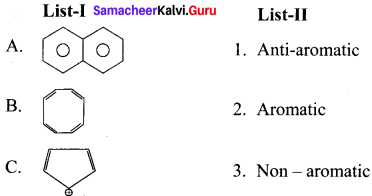

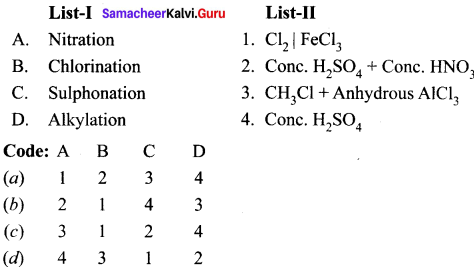
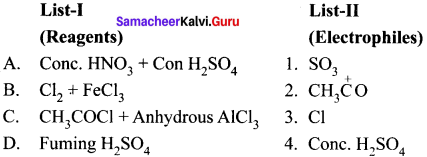

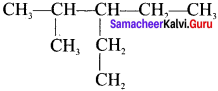
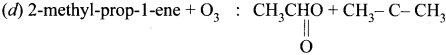
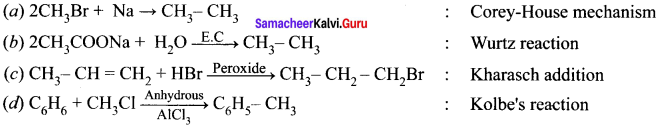
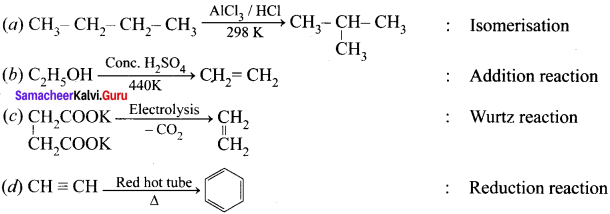
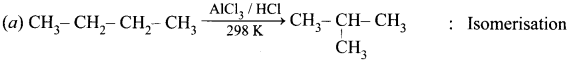
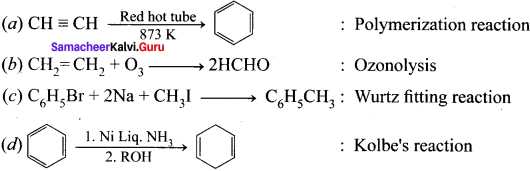
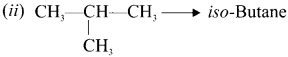

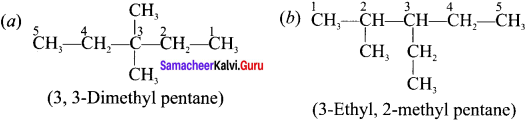
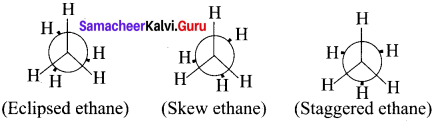
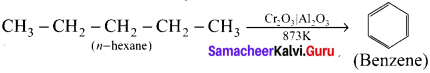
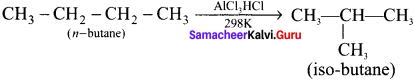
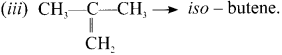

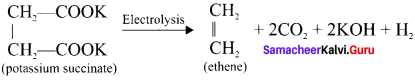
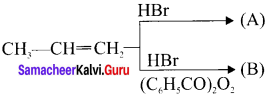
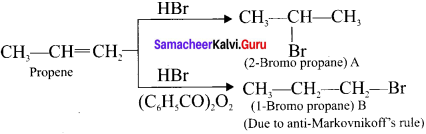
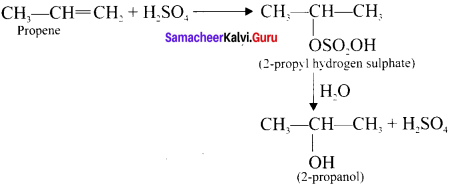
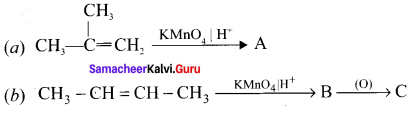
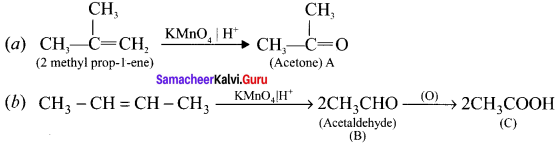
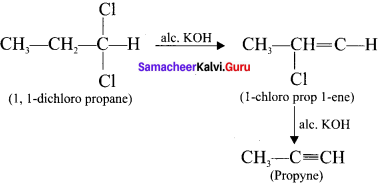
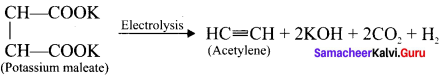
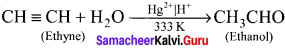

 Cyclopropyl cation.
Cyclopropyl cation.