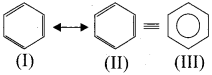
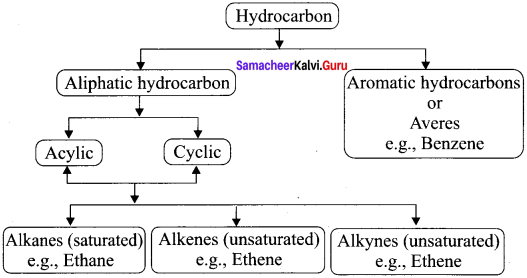
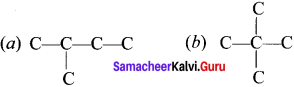
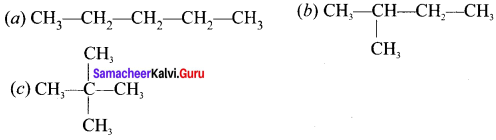
Students who are in search of 11th Bio Botany exam can download this Tamilnadu State Board Solutions for 11th Bio Botany Chapter 7 Cell Cycle from here for free of cost. These cover all Chapter 7 Cell Cycle Questions and Answers, PDF, Notes, Summary. Download the Samacheer Kalvi 11th Biology Book Solutions Questions and Answers by accessing the links provided here and ace up your preparation.
Tamilnadu Samacheer Kalvi 11th Bio Botany Solutions Chapter 7 Cell Cycle
Kickstart your preparation by using this Tamilnadu State Board Solutions for 11th Bio Botany Chapter 7 Cell Cycle Questions and Answers get the max score in the exams. You can cover all the topics of Chapter 7 Cell Cycle Questions and Answers easily after studying the Tamilnadu State Board 11th Bio Botany Textbook solutions pdf.
Samacheer Kalvi 11th Bio Botany Cell Cycle Text Book Back Questions and Answers
I. Multiple Choice Questions
Choose the correct answer
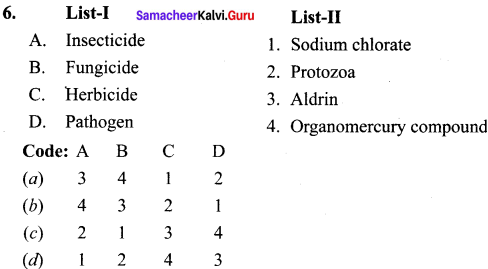
Question 1.
The correct sequence in cell cycle is …………… .
(a) S-M-G1-G2
(b) S-G1-G2-M
(c) G1-S-G2-M
(d) M-G-G2-S
Answer:
(c) G1-S-G2-M
Question 2.
If mitotic division is restricted in G1 phase of the cell cycle then the condition is known as …………… .
(a) S Phase
(b) G2 Phase
(c) M Phase
(d) G0 Phase
Answer:
(d) G0 Phase
Question 3.
Anaphase promoting complex APC is a protein degradation machinery necessary for proper mitosis of animal cells. If APC is defective in human cell, which of the following is expected to occur?
(a) Chromosomes will be fragmented
(b) Chromosomes will not condense
(c) Chromosomes will not segregate
(d) Recombination of chromosomes will occur
Answer:
(b) Chromosomes will not condense
Question 4.
In S phase of the cell cycle …………… .
(a) Amount of DNA doubles in each cell
(b) Amount of DNA remains same in each cell
(c) Chromosome number is increased
(d) Amount of DNA is reduced to half in each cell
Answer:
(a) Amount of DNA doubles in each cell
Question 5.
Centromere is required for …………… .
(a) Transcription
(b) Crossing over
(c) Cytoplasmic cleavage
(d) Movement of chromosome towards pole
Answer:
(d) Movement of chromosome towards pole
Question 6.
Synapsis occur between …………… .
(a) mRNA and ribosomes
(b) Spindle fibres and centromeres
(c) Two homologous chromosomes
(d) A male and a female gamete
Answer:
(c) Two homologous chromosomes
Question 7.
In meiosis crossing over is initiated at …………… .
(a) Diplotene
(b) Pachytene
(c) Leptotene
(d) Zygotene
Answer:
(b) Pachytene
Question 8.
Colchicine prevents the mitosis of the cells at which of the following stage …………… .
(a) Anaphase
(b) Metaphase
(c) Prophase
(d) Interphase
Answer:
(b) Metaphase
Question 9.
The paring of homologous chromosomes on meiosis is known as …………… .
(a) Bivalent
(b) Synapsis
(c) Disjunction
(d) Synergids
Answer:
(b) Synapsis
Question 10.
Anastral mitosis is the characteristic feature of …………… .
(a) Lower animals
(b) Higher animals
(c) Higher plants
(d) All living organisms
Answer:
(c) Higher plants
Question 11.
Write any three significance of mitosis.
Answer:
Exact copy of the parent cell is produced by mitosis (genetically identical).
- Genetic stability – daughter cells are genetically identical to parent cells.
- Repair of tissues – damaged cells must be replaced by identical new cells by mitosis.
- Regeneration – Arms of star fish.
Question 12.
Differentiate between Mitosis and Meiosis.
Answer:
Difference Between Mitosis and Meiosis:
|
Difference Between Mitosis and Meiosis |
|
| Mitosis |
Meiosis |
| 1. One division | 1. Two divisions |
| 2. Number of chromosomes remains the same | 2. Number of chromosomes is halved |
| 3. Homologous chromosomes line up separately on the metaphase plate | 3. Homologous chromosomes line up in pairs at the metaphase plate |
| 4. Homologous chromosome do not pair up | 4. Homologous chromosome pairup to form bivalent |
| 5. Chiasmata do not form and crossing over never occurs | 5. Chiasmata form and crossingover occurs |
| 6. Daughter cells are genetically identical | 6. Daughter cells are genetically different from the parent cells |
| 7. Two daughter cells are formed | 7. Four daughter cells are formed |
Question 13.
Given an account of G0 phase.
Answer:
Some cells exit G1 and enters a quiescent stage called G0, where the cells remain metabolically active without proliferation. Cells can exist for long periods in G0 phase. In G0 cells cease growth with reduced rate of RNA and protein synthesis. The G0 phase is not permanent. Mature neuron and skeletal muscle cell remain permanently in G0 .Many cells in animals remains in G0 unless called onto proliferate by appropriate growth factors or other extracellular signals. G0 cells are not dormant.
Question 14.
Differentiate Cytokinesis in plant cells and animal cells.
Answer:
1. Cytokinesis in Plant Cells:
Division of the cytoplasm often starts during telophase. In plants, cytokinesis cell plate grows from centre towards lateral walls centrifugal manner of cell plate formation. Phragmoplast contains microtubules, actin filaments and vesicles from golgi apparatus and ER. The golgi vesicles contains carbohydrates such as pectin, hemicellulose which move along the microtubule of the pharagmoplast to the equator fuse, forming a new plasma membrane and the materials which are placed their becomes new cell wall.
The first stage of cell wall construction is a line dividing the newly forming cells called a cell plate. The cell plate eventually stretches right across the cell forming the middle lamella. Cellulose builds up on each side of the middle lamella to form the cell walls of two new plant cells.
2. Cytokinesis in Animal Cells:
It is a contractile process. The contractile mechanism contained in contractile ring located inside the plasma membrane. The ring consists of a bundle of microfilaments assembled from actin and myosin. This fibril helps for the generation of a contractile force. This force draws the contractile ring inward forming a cleavage furrow in the cell surface dividing the cell into two.
Question 15.
Write about Pachytene and Diplotene of Prophase I.
Answer:
1. Pachytene: At this stage bivalent chromosomes are clearly visible as tetrads. Bivalent of meiosis I consists of 4 chromatids and 2 centromeres. Synapsis is completed and recombination nodules appear at a site where crossing over takes place between non – sister chromatids of homologous chromosome. Recombination of homologous chromosomes is completed by the end of the stage but the chromosomes are linked at the sites of crossing over. This is mediated by the enzyme recombinase.
2. Diplotene: Synaptonemal complex disassembled and dissolves. The homologous chromosomes remain attached at one or more points where crossing over has taken place. These points of attachment where ‘X’ shaped structures occur at the sites of crossing over is called Chiasmata. Chiasmata are chromatin structures at sites where recombination has been taken place. They are specialised chromosomal structures that hold the homologous chromosomes together.
Sister chromatids remain closely associated whereas the homologous chromosomes tend to separate from each other but are held together by chiasmata. This substage may last for days or years depending on the sex and organism. The chromosomes are very actively transcribed in females as the egg stores up materials for use during embryonic development. In animals, the chromosomes have prominent loops called lampbrush chromosome.
Samacheer Kalvi 11th Bio Botany Cell Cycle Additional Questions and Answers
I. Multiple Choice Questions
Choose the correct answer:
Question 1.
Most of the neurons in the brain are in …………… stage.
(a) G1
(b) S
(c) G2
(d) G0
Answer:
(d) G0
Question 2.
Short, constricted region in the chromosome is …………… .
(a) Kinetochore
(b) Centromere
(c) Satellite
(d) Telomere
Answer:
(b) Centromere
Question 3.
Robert Brown discovered the nucleus in the cells of …………… roots.
(a) Mirabilas
(b) Orchid
(c) Moringa
(d) Oryza
Answer:
(b) Orchid
Question 4.
Scientist who described chromosomes for the first time is …………… .
(a) Robert Brown
(b) Anton van Leeuwenhoek
(c) Boveri
(d) Anton Schneider
Answer:
(d) Anton Schneider
Question 5.
Number of chromosomes in onion cell is …………… .
(a) 8
(b) 16
(c) 32
(d) 64
Answer:
(a) 16
Question 6.
Longest part of the cell cycle is …………… .
(a) Prophase
(b) G1 Phase
(c) Interphase
(d) Sphase
Answer:
(c) Interphase
Question 7.
Eukaryotic cells divides every …………… .
(a) 12
(b) 24
(c) 1
(d) 6
Answer:
(b) 24
Question 8.
Cell cycle was discovered by …………… .
(a) Singer & Nicolson
(b) Prevost & Dumans
(c) Schleider & Schwann
(d) Boveri
Answer:
(b) Prevost & Dumans
Question 9.
G0 stage is called as …………… stage.
(a) Quiescent
(b) Metabolically active
(c) Synthesis of DNA
(d) Replication
Answer:
(a) Quiescent
Question 10.
…………… protein acts as major check point in phase.
(a) Porins
(b) Kinases
(c) Cyclins
(d) Ligases
Answer:
(c) Cyclins
Question 11.
Replication of DNA occurs at …………… phase.
(a) G0
(b) G1
(c) S
(d) G2
Answer:
(c) S
Question 12.
Condensation of interphase chromosomes into mitotic forms is done by …………… proteins.
(a) MPF
(b) APF
(c) AMF
(d) MAF
Answer:
(a) MPF
Question 13.
Which of the following is also called as direct division?
(a) Amitosis
(b) Meiosis
(c) Mitosis
(d) Reduction division
Answer:
(a) Amitosis
Question 14.
Cells of mammalian cartilage undergoes …………… .
(a) Amitosis
(b) Meiosis
(c) Mitosis
(d) Equational division
Answer:
(a) Amitosis
Question 15.
Yeast cells undergo …………… .
(a) Open mitosis
(b) Closed mitosis
(c) Amitosis
(d) Meiosis
Answer:
(b) Closed mitosis
Question 16.
…………… is the longest phase in mitosis.
(a) Anaphase
(b) Telophase
(c) Prophase
(d) Interphase
Answer:
(c) Prophase
Question 17.
The DNA protein complex present in the centromere is …………… .
(a) Cyclin
(b) Kinesis
(c) MPF
(d) Kinetochore
Answer:
(d) Kinetochore
Question 18.
…………… protein induces the break down of cohesion proteins leading to chromatid separation during mitosis.
(a) APC
(b) MPF
(c) Cyclin
(d) Kinetochore
Answer:
(a) APC
Question 19.
Regeneration of arms of star fish is due to …………… .
(a) Meiosis
(b) Amitosis
(c) Mitosis
(d) Budding
Answer:
(c) Mitosis
Question 20
…………… is called as reduction division.
(a) Meiosis
(b) Mitosis
(c) Amitosis
(d) Budding
Answer:
(a) Meiosis
Question 21.
Bivalents occur at …………… stage.
(a) Zygotene
(b) Leptotene
(c) Pachytene
(d) Diplotene
Answer:
(a) Zygotene
Question 22.
Recombination of chromosomes occur at …………… .
(a) Zygotene
(b) Leptotene
(c) Pachytene
(d) Diplotene
Answer:
(c) Pachytene
Question 23.
Terminalisation of chiasmata occurs at …………… .
(a) Zygotene
(b) Leptotene
(c) Diakinesis
(d) Pachytene
Answer:
(c) Diakinesis
Question 24.
Number of daughter cells formed at the end of Meiosis I is …………… .
(a) 2
(b) 4
(c) 1
(d) 0
Answer:
(a) 2
Question 25.
…………… division leads to genetic variability.
(a) Mitotic
(b) Amitotic
(c) Meiotic
(d) Equational
Answer:
(c) Meiotic
Question 26.
Crossing over occurs at …………… stage.
(a) Leptotene
(b) Zygotene
(c) Pachytene
(d) Diplotene
Answer:
(c) Pachytene
Question 27.
Which of the following is not a mitogen?
(a) Giberellin
(b) Ethylene
(c) Kinetin
(d) Colchicine
Answer:
(d) Colchicine
Question 28.
In plants mitosis occurs at …………… cells.
(a) Sclerenchyma
(b) Meristem
(c) Xylem
(d) Parenchyma
Answer:
(b) Meristem
Question 29.
Which of the following alone is formed in the division of plant cells?
(a) Aster
(b) Centrioles
(c) Spindle
(d) Microtubules
Answer:
(c) Spindle
Question 30.
Amphiastral type cell division is seen in …………… cells.
(a) Fungal
(b) Algal
(c) Plant cells
(d) Animal
Answer:
(d) Animal
II. Very Short Answer Type Questions (2 Marks)
Question 1.
Name the two types of nuclear division.
Answer:
The two types of nuclear division:
- Mitosis and
- Meiosis.
Question 2.
Define Cell Cycle.
Answer:
A series of events leading to the formation of new cell is known as cell cycle.
Question 3.
Who discovered the Cell Cycle?
Answer:
Prevost & Dumans in 1824.
Question 4.
Draw a tabular column showing the duration of various phase in the cell cycle of human cell.
Answer:
A tabular column showing the duration of various phase in the cell cycle of human cell:
|
Cell cycle of a proliferating human cell |
|
|
Phase |
Time Duration (in hrs) |
| 1. G2 | 1. 11 |
| 2. S | 2. 8 |
| 3. G2 | 3. 4 |
| 4. M | 4. 1 |
Question 5.
Define C – Value.
Answer:
C – Value is the amount in picograms of DNA contained within a haploid nucleus.
Question 6.
Which is the longest phase of cell cycle? What happens during that phase?
Answer:
Interphase is the longest phase. Cells are metabolically active and involved in protein synthesis and growth.
Question 7.
Name the phases which comprises the Interphase.
Answer:
The phases which comprises the Interphase:
- G1 Phase
- S Phase and
- G2 Phase.
Question 8.
Name the proteins involved in the activation of genes & their proteins to perform cell division.
Answer:
Kinases & Cyclins.
Question 9.
What do you mean by G0 stage?
Answer:
G0 stage is called as quiescent stage, where the cells remain metabolically active without proliferation.
Question 10.
What is the role of MPF in Cell cycle?
Answer:
Maturation Promoting Factor (MPF) brings about condensation of interphase chromosomes into the mitotic form.
Question 11.
Distinguish between Karyokinesis & Cytokinesis.
Answer:
Between Karyokinesis & Cytokinesis:
- Karyokinesis: Karyokinesis refers to the nuclear division.
- Cytokinesis: Cytokinesis refers to the cytoplasmic division.
Question 12.
Point out any two cell – types which remain G0 phase.
Answer:
Mature neurons and Skeletal muscle cells.
Question 13.
Why amitosis is called as incipient cell division?
Answer:
Amitosis is also called incipient cell division. Since there is no spindle formation and chromatin material does not condense.
Question 14.
List out the disadvantages of Amitosis.
Answer:
The disadvantages of Amitosis:
- Causes unequal distribution of chromosomes.
- Can lead to abnormalities in metabolism and reproduction.
Question 15.
Mitosis also called as equational division – Justify.
Answer:
At the end of mitosis the number of chromosomes in the parent and the daughter (Progeny) cells remain the same so it is also called as equational division.
Question 16.
Enumerate the stages of mitosis.
Answer:
Mitosis is divided into four stages prophase, metaphase, anaphase and telophase.
Question 17.
Define an aster.
Answer:
In animal cell the centrioles extend a radial array of microtubules towards the plasma membrane when they reach the poles of the cell. This arrangement of microtubules is called an aster. Plant cells do not form asters.
Question 18.
What is metaphase plate?
Answer:
The alignment of chromosome into compact group at the equator of the cell is known as metaphase plate.
Question 19.
What is Kinetochore?
Answer:
Kinetochore is a DNA – Protein complex present in the centromere DNA, where the microtubules are attached. It is a trilaminar disc like plate.
Question 20.
How will you calculate the length of the S period.
Answer:
Length of the S period = Fraction of cells in DNA replication × generation time.
Question 21.
Which type of cell division occurs in reproductive cells? What will be the result?
Answer:
Meiosis takes place in the reproductive organs. It results in the formation of gametes with half the normal chromosome number.
Question 22.
Define Synapsis.
Answer:
In Zygotene, pairing of homologous chromosomes takes place and it is known as synapsis.
Question 23.
What do you understand by independent assortment?
Answer:
The random distribution of homologous chromosomes in a cell in Metaphase I is called independent assortment.
Question 24.
Define Mitogen. Give an example.
Answer:
The factors which promote cell cycle proliferation is called mitogen.
Example: gibberellin. These increase mitotic rate.
Question 25.
What are mitotic poisons.
Answer:
Certain chemical components act as inhibitors of the mitotic cell division and they are called mitotic poisons.
Question 26.
Distinguish between Anastral & Amphiastral.
Answer:
Between Anastral & Amphiastral:
Anastral:
- This is present only in plant cells.
- No asters or centrioles are formed only spindle fibres are formed during cell division.
Amphiastral:
- This is found in animal cells.
- Aster and centrioles are formed at each pole of the spindle during cell division.
Question 27.
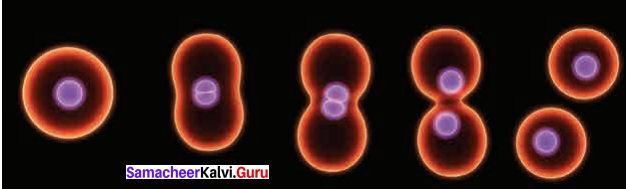
Draw a simple diagram to show the Amitosis.
Answer:
The Amitosis:
III. Short Answer Type Questions (3 Marks)
Question 1.
What is the role of nucleus in the cell?
Answer:
The role of nucleus in the cell:
- Control activities of the cell.
- Genetic information copied from cell to cell while the cell divides.
- Hereditary characters are passed onto new individuals when gametic cells fuse together in sexual reproduction.
Question 2.
What are restriction points? Mention its role in Cell cycle.
Answer:
The checkpoint called the restriction point at the end of G1 it determines a cells fate whether it will continue in the cell cycle and divide or enter a stage called G0 as a quiescent stage and probably as specified cell or die.
Question 3.
Point out the reasons responsible for the arresting of the cell in G1 phase?
Answer:
Cells are arrested in G1 due to:
- Nutrient deprivation
- Lack of growth factors or density dependant inhibition
- Undergo metabolic changes and enter into G0 state.
Question 4.
Write a note on G0 phase.
Answer:
Some cells exit G1 and enters a quiescent stage called G0, where the cells remain metabolically active without proliferation. Cells can exist for long periods in G0 phase. In G0 cells cease growth with reduced rate of RN A and protein synthesis. The G0 phase is not permanent. Mature neuron and skeletal muscle cell remain permanently in G0. Many cells in animals remains in G0 unless called onto proliferate by appropriate growth factors or other extracellular signals. G0 cells are not dormant.
Question 5.
List out the events taking place in S – Phase.
Answer:
S Phase – Synthesis phase – cells with intermediate amounts of DNA Growth of the cell continues as replication of DNA occur, protein molecules called histones are synthesised and attach to the DNA. The centrioles duplicate in the cytoplasm. DNA content increases from 2C to 4C.
Question 6.
Distinguish between Karyokinesis & Cytokinesis.
Answer:
Karyokinesis:
- Involves division of nucleus.
- Nucleus develops a constriction at the center and becomes dumbellshaped.
- Constriction deepens and divides the nucleus into two.
Cytokinesis:
- Involves division of cytoplasm.
- Plasma membrane develops a constriction along nuclear constriction.
- It deepens centripetally and finally divides the cell into two cells.
Question 7.
Explain the differences between closed and open mitosis.
Answer:
Between closed and open mitosis:
- In closed mitosis, the nuclear envelope remains intact and chromosomes migrate to opposite poles of a spindle within the nucleus. Example: Yeast and slime molds.
- In open mitosis, the nuclear envelope breaks down and then reforms around the 2 sets of separated chromosome. Example: Most plants and animals cells.
Question 8.
What happens to plant cells at the end of Telophase in Mitosis?
Answer:
In plants, phragmoplast are formed between the daughter cells. Cell plate is formed between the two daughter cells, reconstruction of cell wall takes place. Finally the cells are separated by the distribution of organelles, macromolecules into two newly formed daughter cells.
Question 9.
Bring out the significance of Meiosis.
Answer:
The significance of Meiosis:
- Meiosis maintains a definite constant number of chromosomes in organisms.
- Crossing over takes place and exchange of genetic material leads to variations among species. These variations are the raw materials to evolution. Meiosis leads to genetic variability by partitioning different combinations of genes into gametes through independent assortment.
- Adaptation of organisms to various environmental stress.
Question 10.
Differentiate between the mitosis of Plant Cell & Animal Cell.
Answer:
Plants:
- Centrioles are absent
- Asters are not formed
- Cell division involves formation of a cell plate
- Occurs mainly at meristem.
Animals:
- Centrioles are present
- Asters are formed
- Cell division involves furrowing and cleavage of cytoplasm
- Occurs in tissues throughout the body.
Question 11.
Explain briefly about Endomitosis.
Answer:
The replication of chromosomes in the absence of nuclear division and cytoplasmic division resulting in numerous copies within each cell is called endomitosis. Chromonema do not separate to form chromosomes, but remain closely associated with each other. Nuclear membrane does not rupture. So no spindle formation. It occurs notably in the salivary glands of Drosophila and other flies. Cells in these tissues contain giant chromosomes (polyteny), each consisting of over thousands of intimately associated, or synapsed, chromatids. Example: Polytene chromosome.
Question 12.
How G0 cells help in Closing Technology?
Answer:
Since the DNA of cells in G0, do not replicate. The researcher are able to fuse the donor cells from a sheep’s mammary glands into G0, state by culturing in the nutrient free state. The G0, donor nucleus synchronised with cytoplasm of the recipient egg, which developed into the clone Dolly.
IV. Long Answer Type Questions (5 Marks)
Question 1.
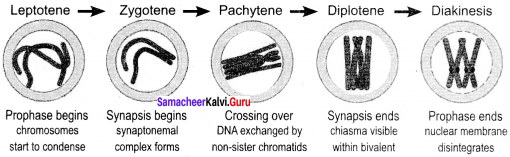
Draw and label the various stages of Prophase I.
Answer:
Label the various stages of Prophase I:
Question 2.
Explain in detail about the various stages of Prophase I.
Answer:
The various stages of Prophase I:
1. Prophase I – Prophase I is of longer duration and it is divided into 5 substages – Leptotene, Zygotene, Pachytene, Diplotene and Diakinesis.
2. Leptotene – Chromosomes are visible under light microscope. Condensation of chromosomes takes place. Paired sister chromatids begin to condense.
3. Zygotene – Pairing of homologous chromosomes takes place and it is known as synapsis. Chromosome synapsis is made by the formation of synaptonemal complex. The complex formed by the homologous chromosomes are called as bivalent (tetrads).
4. Pachytene – At this stage bivalent chromosomes are clearly visible as tetrads. Bivalent of meiosis I consists of 4 chromatids and 2 centromeres. Synapsis is completed and recombination nodules appear at a site where crossing over takes place between non – sister chromatids of homologous chromosome. Recombination of homologous chromosomes is completed by the end of the stage but the chromosomes are linked at the sites of crossing over. This is mediated by the enzyme recombinase.
5. Diplotene – Synaptonemal complex disassembled and dissolves. The homologous chromosomes remain attached at one or more points where crossing over has taken place. These points of attachment where ‘X’ shaped structures occur at the sites of crossing over is called.
6. Chiasmata: Chiasmata are chromatin structures at sites where recombination has been taken place. They are specialised chromosomal structures that hold the homologous chromosomes together. Sister chromatids remain closely associated whereas the homologous chromosomes tend to separate from each other but are held together by chiasmata. This substage may last for days or years depending on the sex and organism. The chromosomes are very actively transcribed in females as the egg stores up materials for use during embryonic development. In animals, the chromosomes have prominent loops called lampbrush chromosome.
7. Diakinesis – Terminalisation of chiasmata. Spindle fibres assemble. Nuclear envelope breaks down. Homologous chromosomes become short and condensed. Nucleolus disappears.
Question 3.
Describe the process of Cytokinesis in Plant cell & Animal Cell.
Answer:
1. Cytokinesis in Plant Cell: Division of the cytoplasm often starts during telophase. In plants, cytokinesis cell plate grows from centre towards lateral walls – centrifugal manner of cell plate formation. Phragmoplast contains microtubules, actin filaments and vesicles from golgi apparatus and ER. The golgi vesicles contains carbohydrates such as pectin, hemicellulose which move along the microtubule of the pharagmoplast to the equator fuse, forming a new plasma membrane and the materials which are placed there becomes new cell wall.
The first stage of cell wall construction is a line dividing the newly forming cells called a cell plate. The cell plate eventually stretches right across the cell forming the middle lamella. Cellulose builds up on each side of the middle lamella to form the cell walls of two new plant cells.
2. Cytokinesis in Animal Cells:
It is a contractile process. The contractile mechanism contained in contractile ring located inside the plasma membrane. The ring consists of a bundle of microfilaments assembled from actin and myosin. This fibril helps for the generation of a contractile force. This force draws the contractile ring inward forming a cleavage furrow in the cell surface dividing the cell into two.
Question 4.
What are the significances of Mitosis.
Answer:
Exact copy of the parent cell is produced by mitosis (genetically identical).
- Genetic stability – Daughter cells are genetically identical to parent cells.
- Growth – As multicellular organisms grow, the number of cells making up their tissue increases. The new cells must be identical to the existing ones.
- Repair of tissues – Damaged cells must be replaced by identical new cells by mitosis.
- Asexual reproduction – Asexual reproduction results in offspring that are identical to the parent. Example Yeast and Amoeba.
- In flowering plants, structure such as bulbs, corms, tubers, rhizomes and runners are produced by mitotic division. When they separate from the parent, they form a new individual. The production of large numbers of offsprings in a short period of time, is possible only by mitosis. In genetic engineering and biotechnology, tissues are grown by mitosis (i.e. in tissue culture).
- Regeneration – Arms of star fish
Question 5.
Explain the various phases in Cell Cycle.
Answer:
The different phases of cell cycle are as follows:
1. Interphase: Longest part of the cell cycle, but it is of extremely variable length. At first glance the nucleus appears to be resting but this is not the case at all. The chromosomes previously visible as thread like structure, have dispersed. Now they are actively involved in protein synthesis, at least for most of the interphase. C – Value is the amount in picograms of DNA contained within a haploid nucleus.
2. G1 Phase: The first gap phase – 2C amount of DNA in cells of G1 The cells become metabolically active and grows by producing proteins, lipids, carbohydrates and cell organelles including mitochondria and endoplasmic reticulum. Many checkpoints control the cell cycle. The checkpoint called the restriction point at the end of G1 it determines a cells fate whether it will continue in the cell cycle and divide or enter a stage called G0 as a quiescent stage and probably as specified cell or die. Cells are arrested in G1 due to:
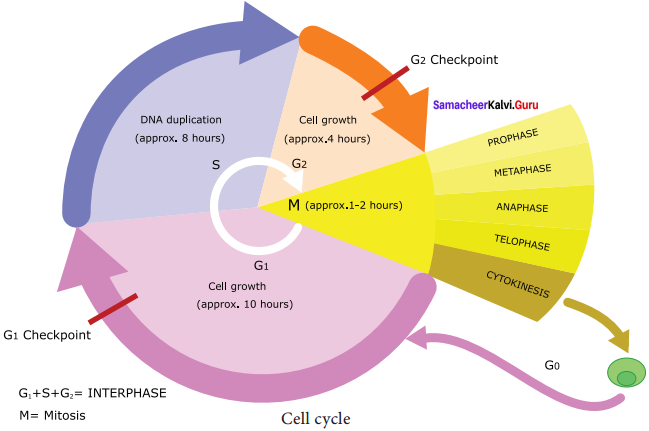
3. Nutrient deprivation: Lack of growth factors or density dependant inhibition. Undergo metabolic changes and enter into G0 state. Biochemicals inside cells activates the cell division. The proteins called kinases and cyclins activate genes and their proteins to perform cell division. Cyclins act as major checkpoint which operates in G1 to determine whether or not a cell divides.
4. G0 Phase: Some cells exit G1 and enters a quiescent stage called G0, where the cells remain metabolically active without proliferation. Cells can exist for long periods in G0 phase. In G0 cells cease growth with reduced rate of RNA and protein synthesis. The G0 phase is not permanent. Mature neuron and skeletal muscle cell remain permanently in G0. Many cells in animals remains in G0 unless called on to proliferate by appropriate growth factors or other extracellular signals. G0 cells are not dormant.
5. S phase – Synthesis phase – cells with intermediate amounts of DNA. Growth of the cell continues as replication of DNA occur, protein molecules called histones are synthesised and attached to the DNA. The centrioles duplicate in the cytoplasm. DNA content increases from 2C to 4C.
6. G2 – The second Gap phase – 4C amount of DNA in cells of G2 and mitosis. Cell growth continues by protein and cell organelle synthesis, mitochondria and chloroplasts divide. DNA content remains as 4C. Tubulin is synthesised and microtubules are formed. Microtubles organise to form spindle fibre. The spindle begins to form and nuclear division follows.
One of the proteins synthesized only in the G2 period is known as Maturation Promoting Factor (MPF). It brings about condensation of interphase chromosomes into the mitotic form. DNA damage checkpoints operates in G1 S and G2 phases of the cell cycle.
Question 6.
List out the important features of Chromosomes.
Answer:
The four important features of the chromosome are:
1. The shape of the chromosome is specific: The long, thin, lengthy structured chromosome contains a short, constricted region called centromere. A centromere may occur any where along the chromosome, but it is always in the same position on any given chromosome. The number of chromosomes per species is fixed: For example the mouse has 40 chromosomes, the onion has 16 and humans have 46.
2. Chromosomes occur in pairs: The chromosomes of a cell occur in pairs, called homologous pairs. One of each pair come originally from each parent. Example, human has 46 chromosomes, 23 coming originally from each parent in the process of sexual reproduction. Chromosomes are copied: Between nuclear divisions, whilst the chromosomes are uncoiled and cannot be seen, each chromosome is copied. The two identical structures formed are called chromatids.
V. Higher Order Thinking Skills (HOTs)
Question 1.
Given that the average duplication time of E.coli is 20 minutes. How much time will two E.coli cells takes to become 32 cells?
Answer:
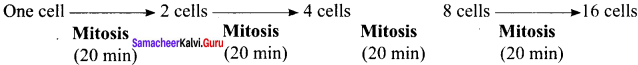
One cells takes 80 minutes to form 16 cells. If 2 cells undergoes division simultaneously, it take 160 minutes (2 hours 40 minutes) to form 32 cells.
Question 2.
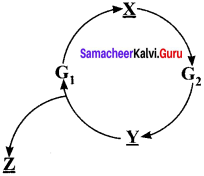
Complete the cell cycle by filling the gaps with respective phases.
Answer:
X= S phase or Synthesis phase
Y= M phase or Mitosis phase
Z= G0 phase
Question 3.
Telophase is reverse of prophase – Comment
Answer:
Events in Prophase:
- Nuclear membrane disappears
- Nucleolus disappear
- Spindle fibre begins to form
- Chromosomes threads condeme to form chromosomes
Events in Telophase:
- Nuclear membrane reappears
- Nucleolus reappears
- Spindle fibre disappears
- Chromosomes decondeme to form chromosomes
Question 4.
Name the pathological condition when uncontrolled cell division occurs.
Answer:
Uncontrolled cell division & abnormal growth of cells leads to the pathological condition called tumor or cancer.
Question 5.
Microspores are produced in the multiples of four, why?
Answer:
Microspores are haploid spores produced from diploid microspores mother cells. Each microspores mother cell (2n) undergoes meiosis producing four Microspores (n). Because a complete meiotic division yields 4 cells. Thus microspores are produced in multiples of four.
Question 6.
Between Prokaryotes & Eukaryotes, which cell has a shorter cell division time.
Answer:
Prokaryotes like bacteria undergo simple form of cell division called binary fission which will get completed with in a hour, whereas Eukaryotic cell division (mitosis) takes nearly 24 hours to get completed. Hence Prokaryotes have shorter cell division time.
Question 7.
Though Prokaryotic cell division differs from Eukaryotic cell division, both show certain common aspects during cell division. Explain.
Answer:
Whether a cell is Prokaryote or Eukaryote, while undergoing division, the following events must occur in common.
- Replication of DNA.
- Cytokinesis at the end of cell division.
Question 8.
An anther has 1204 pollen grains. How many Pollen mother cells must have been there to produce them?Explain.
Answer:
301 – Pollen mother cells: 301 Pollen mother cells undergo meiosis producing 1204 pollen grains. Because at the end of meiosis, each pollen mother cells produces 4 pollen grains.
Question 9.
A cell has 32 chromosomes. It undergoes mitosis. What will be the chromosome number during metaphase?
Answer:
During S phase of interphase, the genetic material of the cell is duplicated. So during metaphase, the chromosome number(chromatid number) will be doubled thus 64 chromosomes (chromatids) will be present.
Question 10.
Why sibilings show disimilarities?
Answer:
Though born to same parents, siblings show dissimilarities and variation due to the crossing over and recombination of chromosomes during meiosis.
Question 11.
Ramu’s met with an accident while riding cycle and got wounded in his leg. After few days, the wound was healed and the skin becomes normal. How?
Answer:
Ramu’s wound was healed because of the mitotic division. As a result of mitosis, new cells are produced and damaged tissues were repaired resulting the damaged skin to become normal.
Question 12.
A flower of tomato plant following the process of sexual reproduction produces 240 viable seeds. What is the minimum number of microspore mother cells involved in this process?
Answer:
60 microspore mother cells are involved in providing 240 pollen grains. Because each microspore mother cell undergoes meiosis producing four pollen grains (i.e. 60 × 4 = 240). Each pollen grain produces two male gametes of which one undergoes true fertilization of ovule producing seeds. Other male gamete participate in double fertilization.
Believing that the Samacheer Kalvi 11th Biology Book Solutions Questions and Answers learning resource will definitely guide you at the time of preparation. For more details about Tamilnadu State 11th Bio Botany Chapter 7 Cell Cycle textbook solutions, ask us in the below comments and we’ll revert back to you asap.







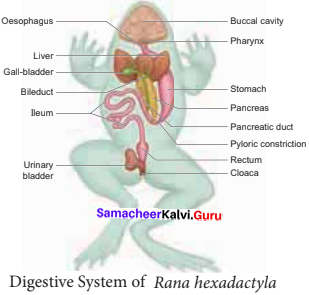
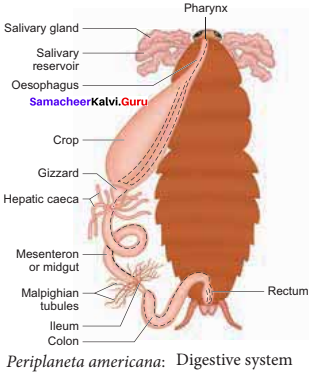
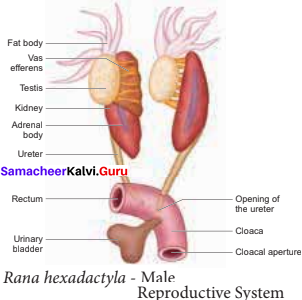
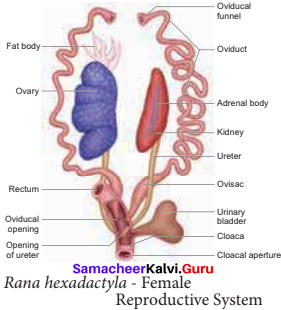


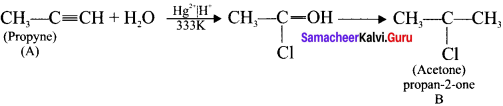
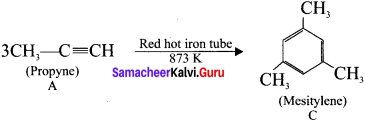
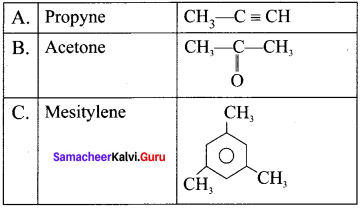
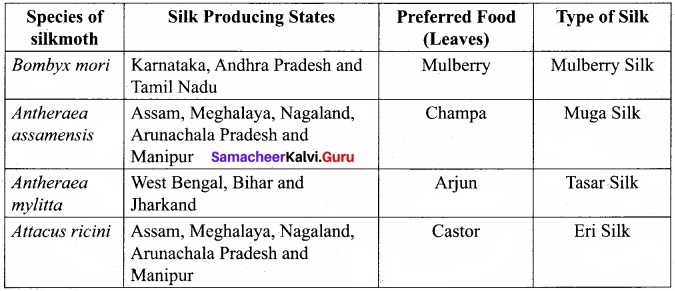
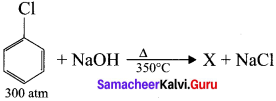
 is ………….
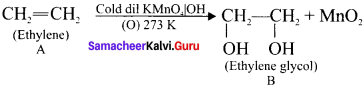
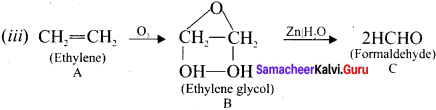
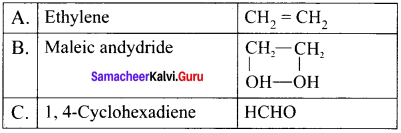
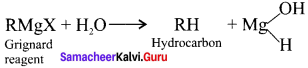
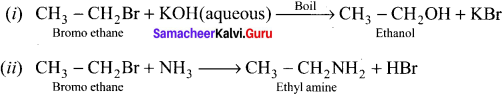
is ………….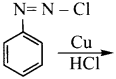 X + N2, X is …………
X + N2, X is …………
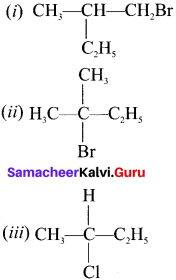

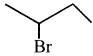
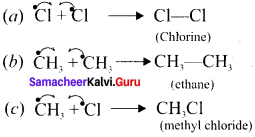
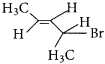
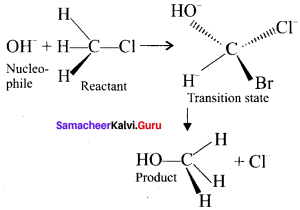
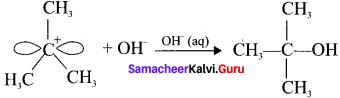
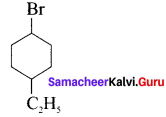
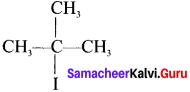
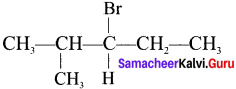
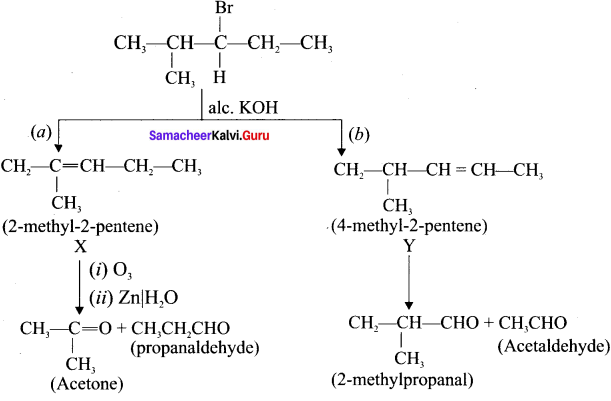
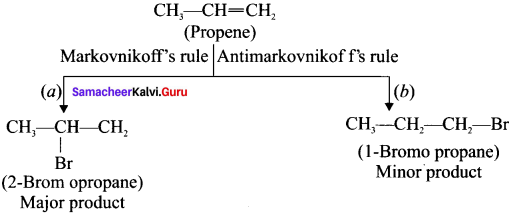
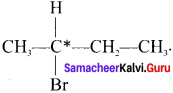 Here the C*is chiral carbon atom and it is surrounded by four different groups. Br
Here the C*is chiral carbon atom and it is surrounded by four different groups. Br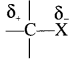
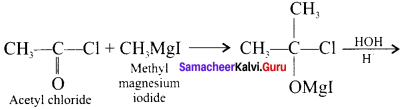
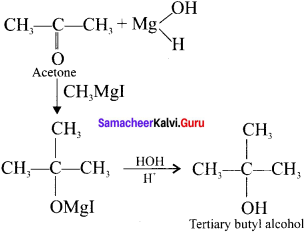
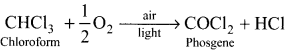
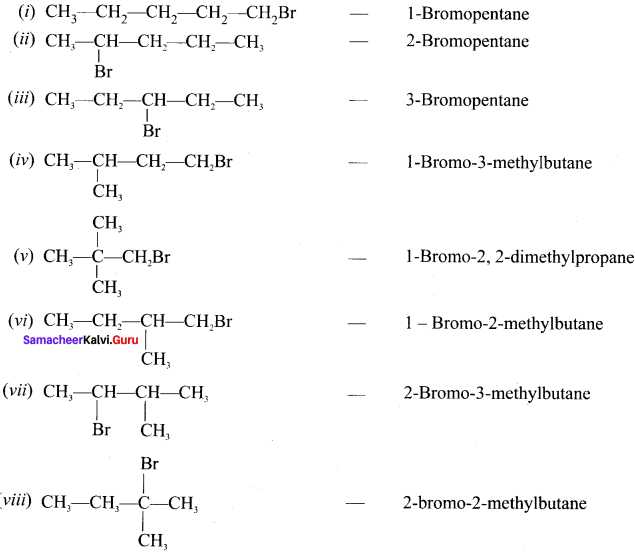
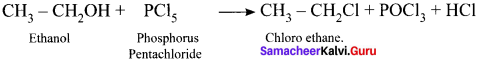
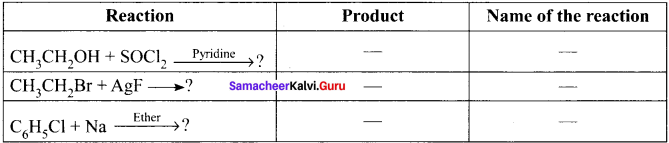
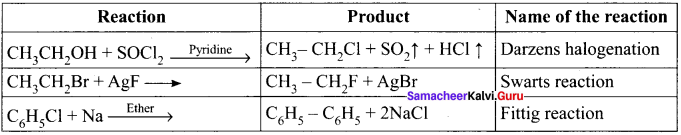
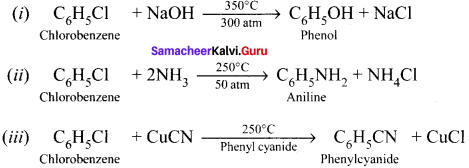


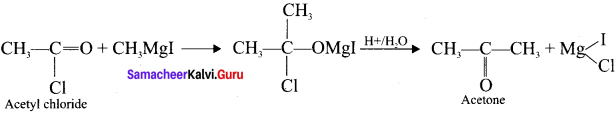


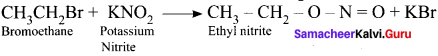
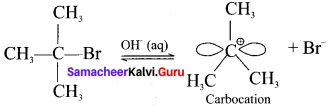
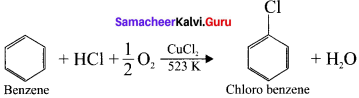
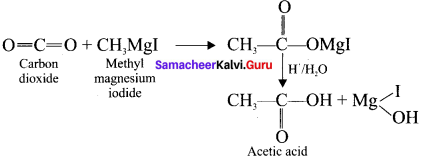
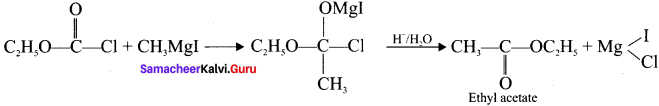
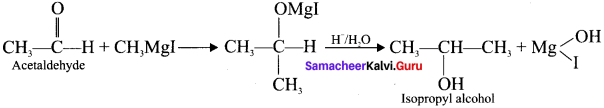
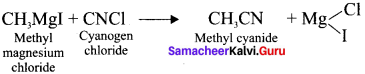
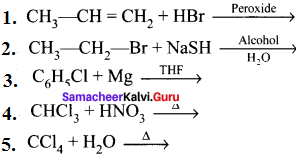
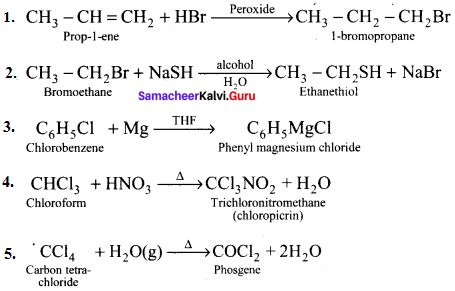
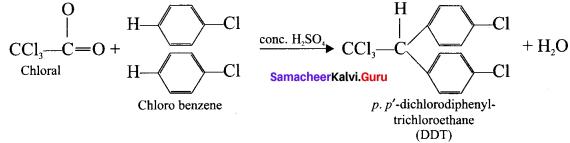
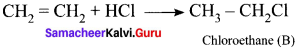
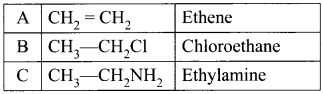
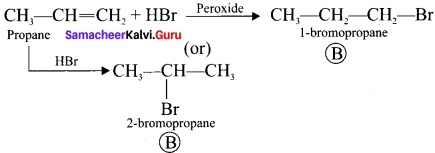
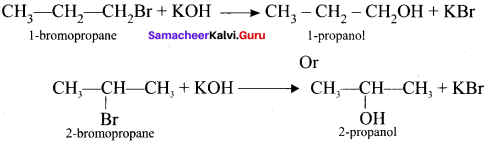
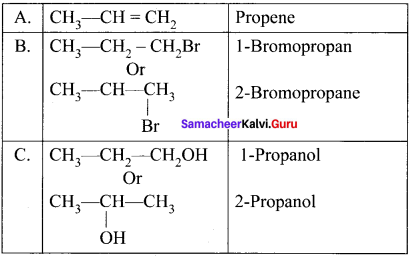
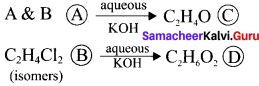
 and
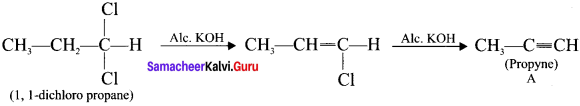
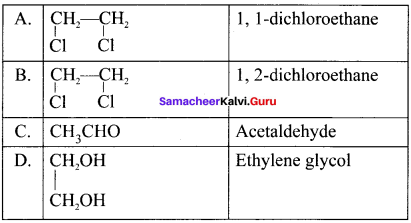
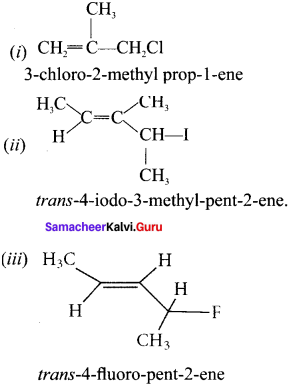
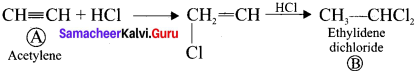
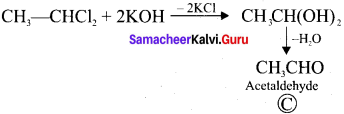
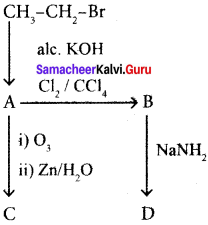
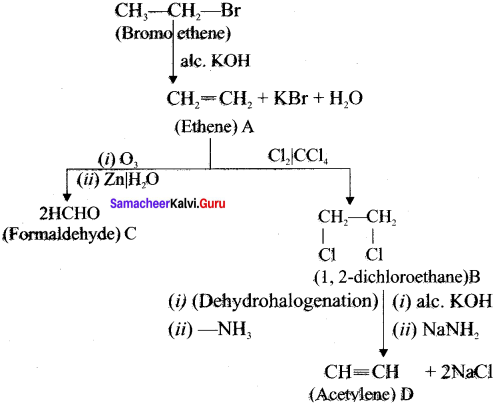
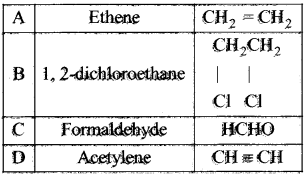
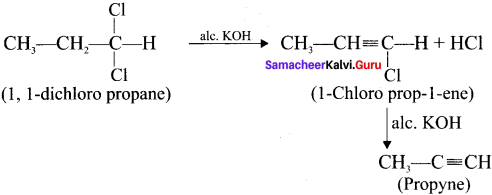
and 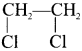 respectively with IUPAC names: 1, 1-dichloroethane (A) and 1.
respectively with IUPAC names: 1, 1-dichloroethane (A) and 1.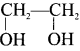 as (D).
as (D).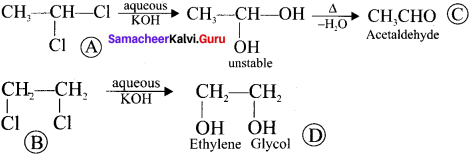
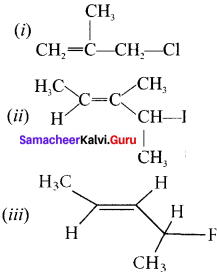
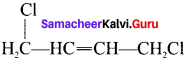
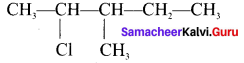
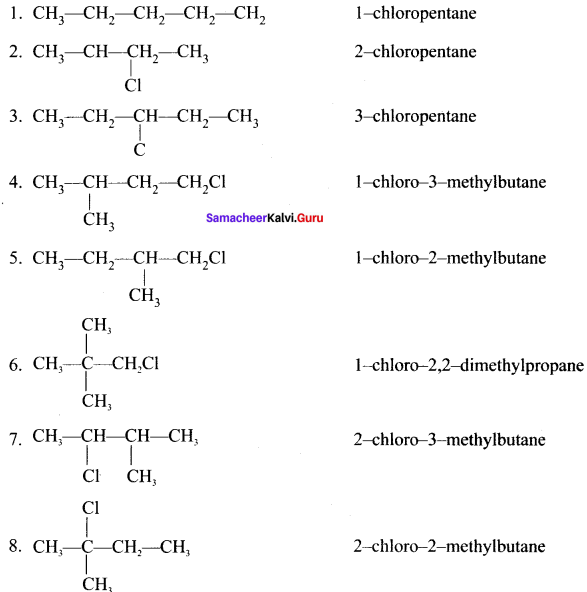
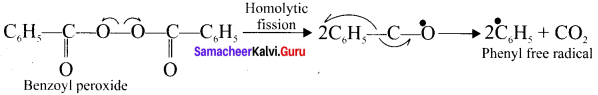
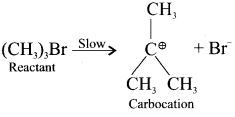
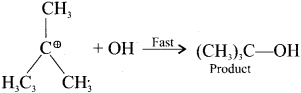
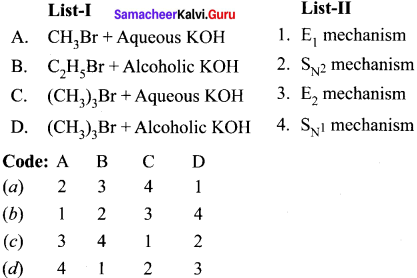
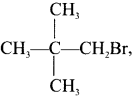
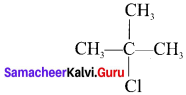
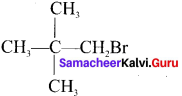
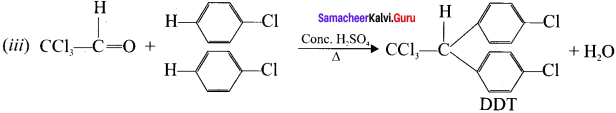
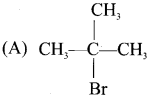
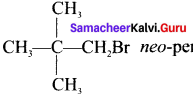 neo-pentyl bromide undergoes nucleophilic substitution reactions very slowly due to steric hindrance of many alkyl groups. When bromide is attached to neo-pentyl carbon atom, the heterolytic cleavage of C-Br takes place very slowly and substitution is also a very slow reaction.
neo-pentyl bromide undergoes nucleophilic substitution reactions very slowly due to steric hindrance of many alkyl groups. When bromide is attached to neo-pentyl carbon atom, the heterolytic cleavage of C-Br takes place very slowly and substitution is also a very slow reaction.
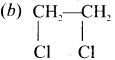
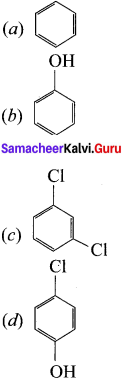
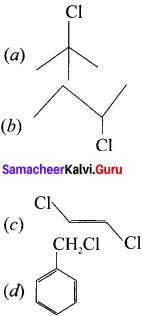
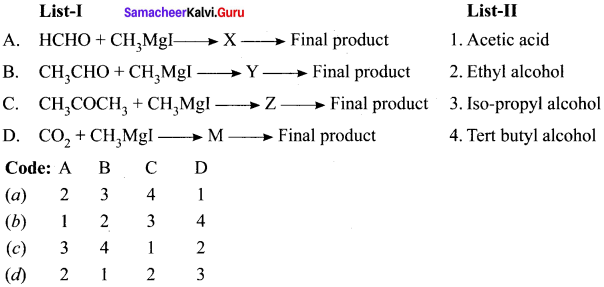
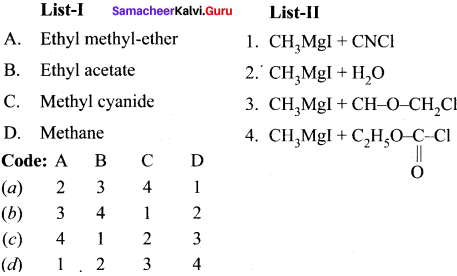

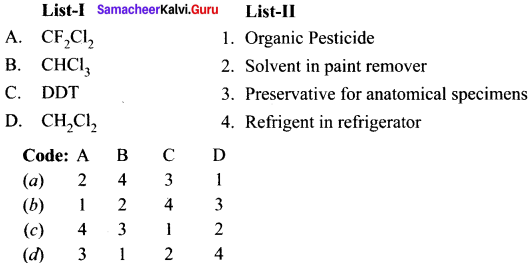
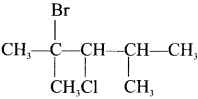 is ………
is ………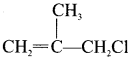 is ………
is ………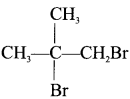 is ………..
is ………..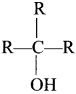
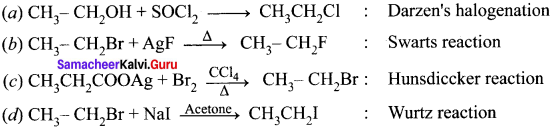
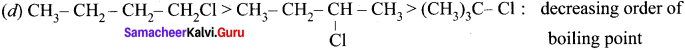
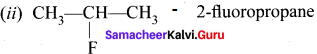
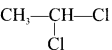
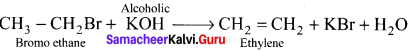
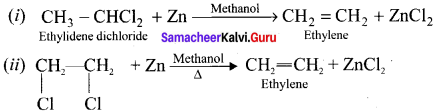
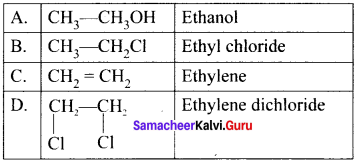
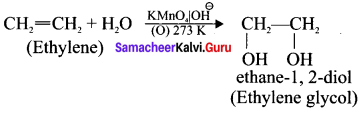
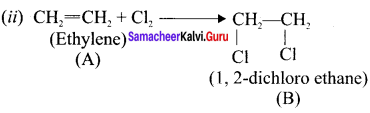
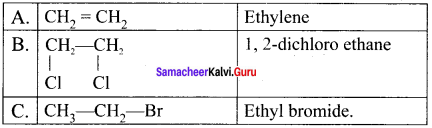
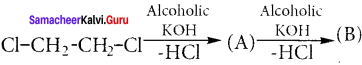
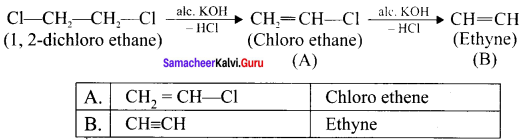
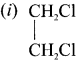 Ethylene dichioride (or) 1, 2-dichioroethane.
Ethylene dichioride (or) 1, 2-dichioroethane.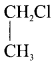 Ethylidene chloride (or) 2-dichioroethane.
Ethylidene chloride (or) 2-dichioroethane.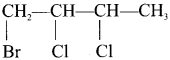
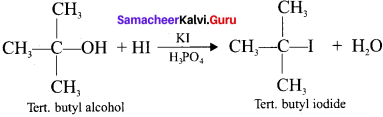

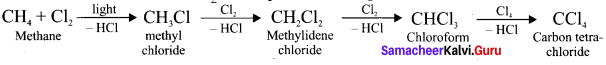
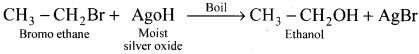
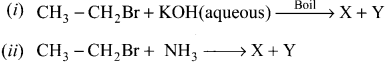

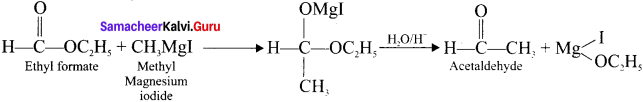
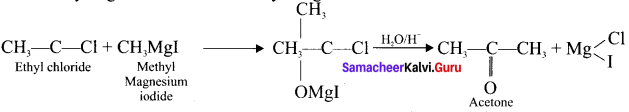
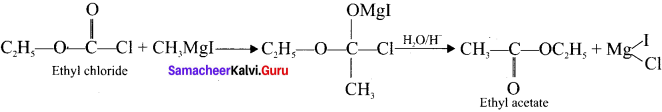

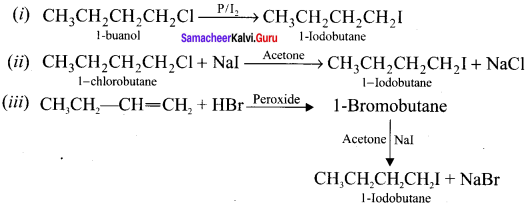
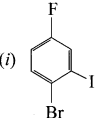 1 – bromo-4-fluoro-2-iodobenzene
1 – bromo-4-fluoro-2-iodobenzene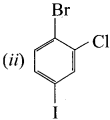 1 – bromo-2-fluoro-4-iodobenzene
1 – bromo-2-fluoro-4-iodobenzene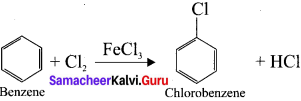
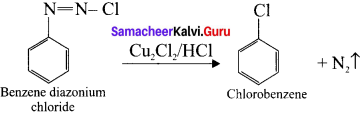
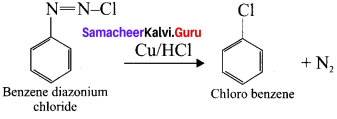


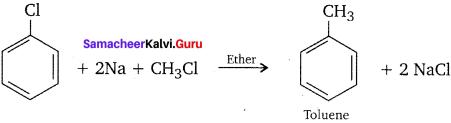
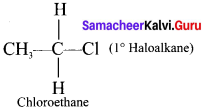
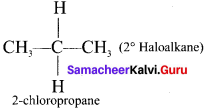
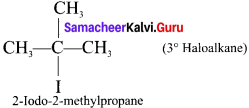
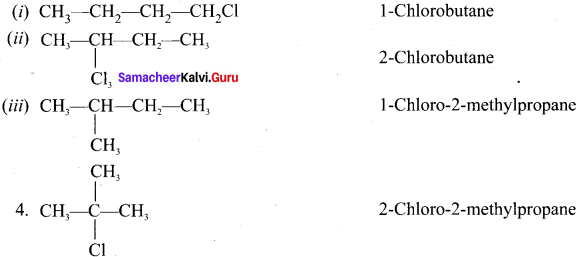
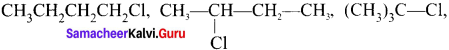
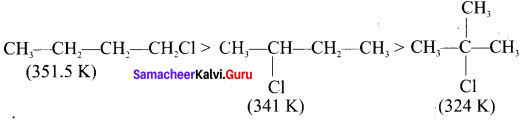
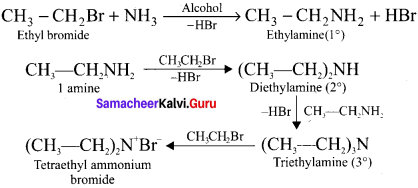

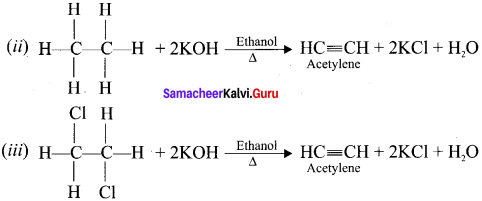
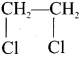 1,2-dichloroethane
1,2-dichloroethane

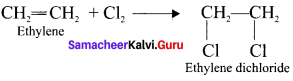
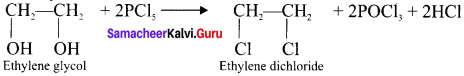
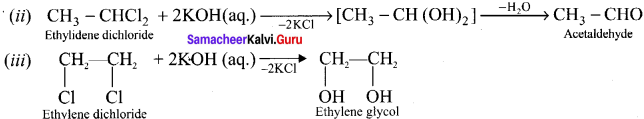
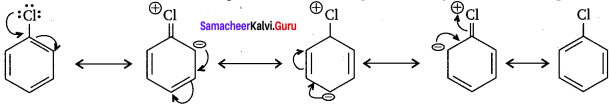
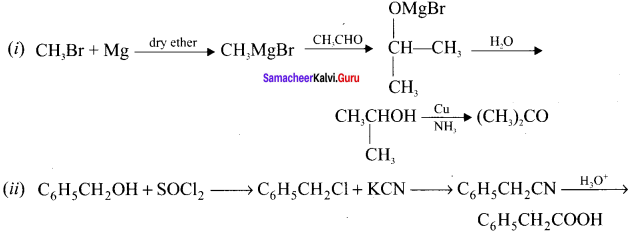
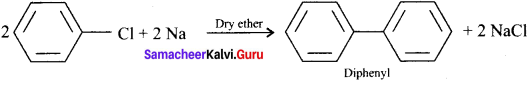
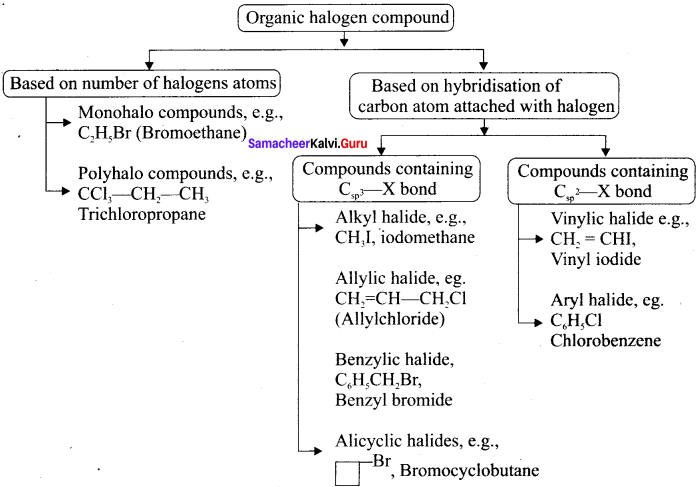
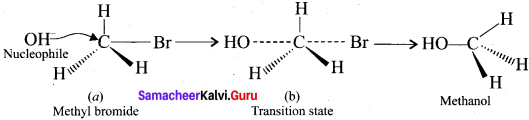
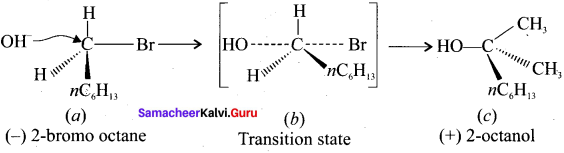

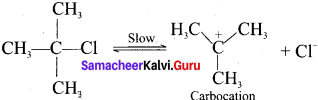
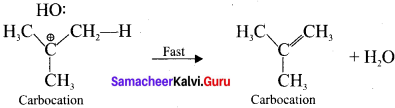
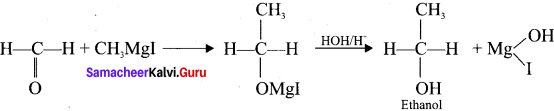
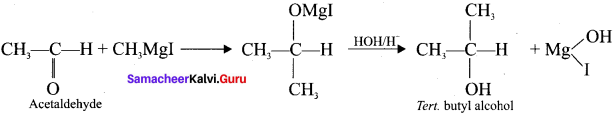
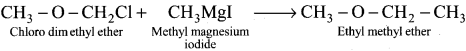
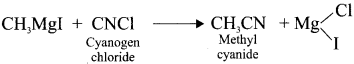

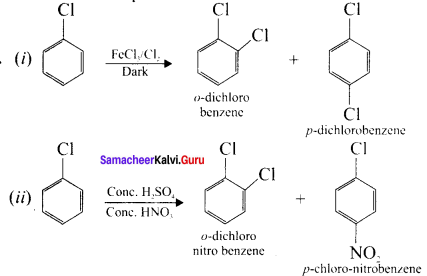
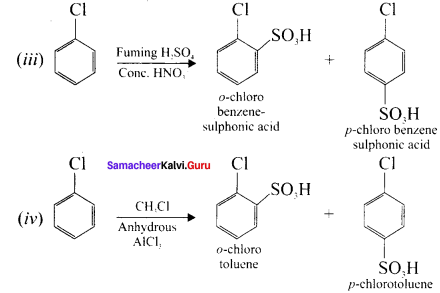


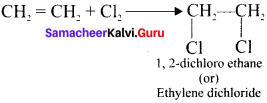
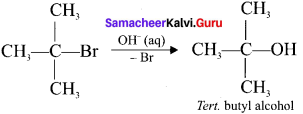
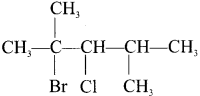
 Tert butyl bromide and
Tert butyl bromide and 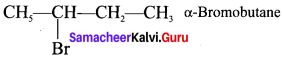
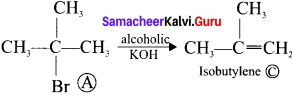
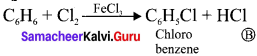
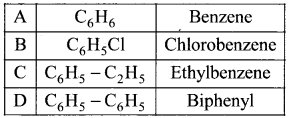
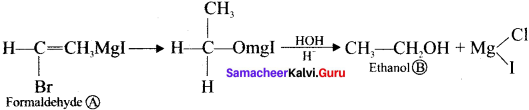
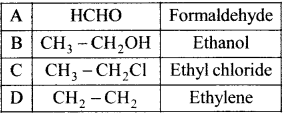
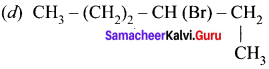
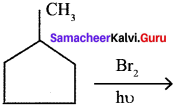

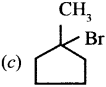
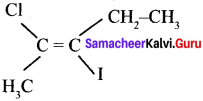
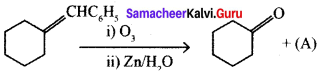
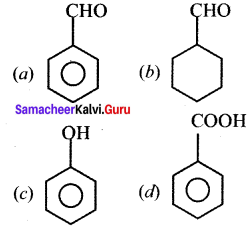
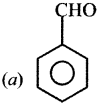
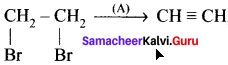 where a is ………
where a is ………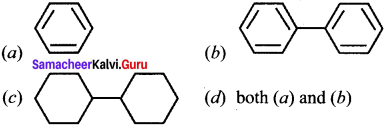
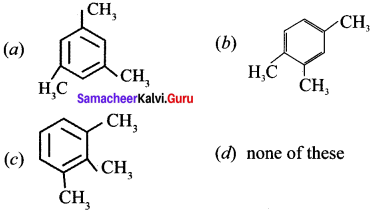
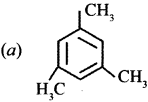

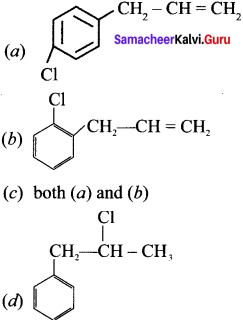
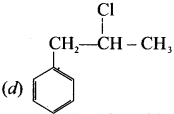
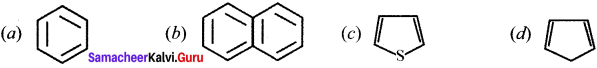
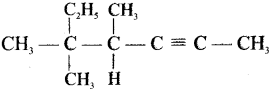
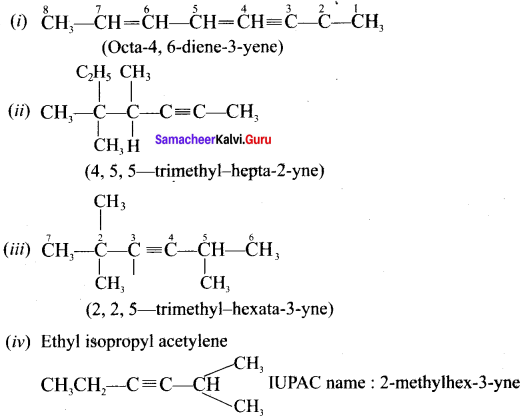
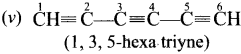
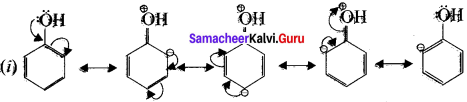
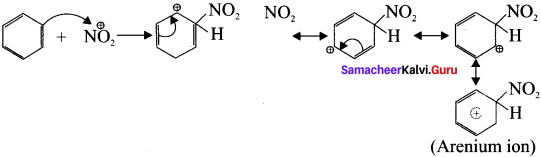
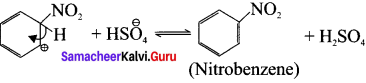

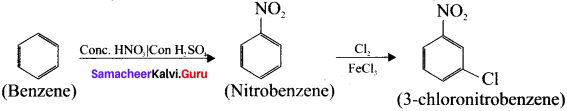
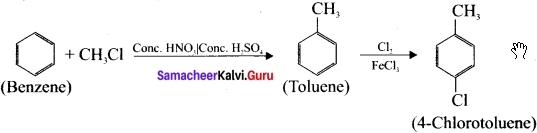
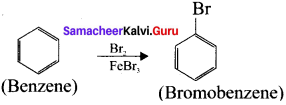
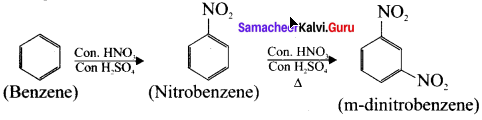
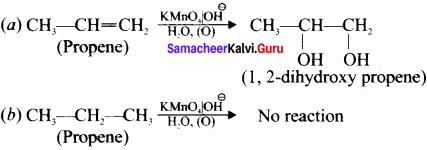
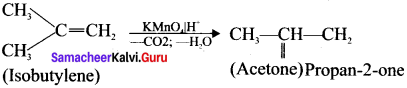
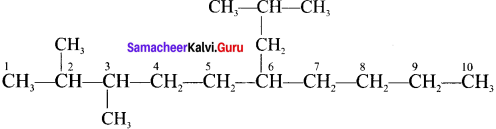
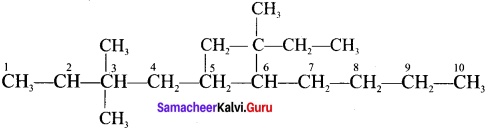
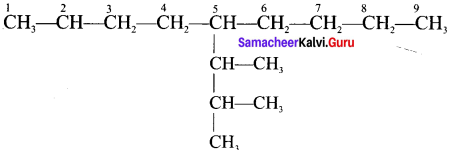
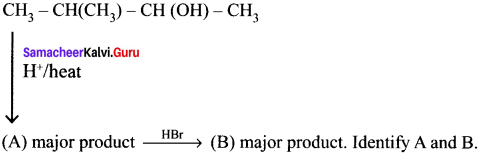
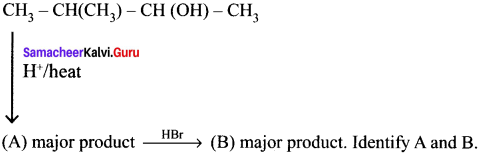
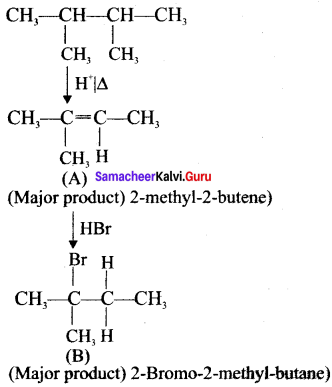
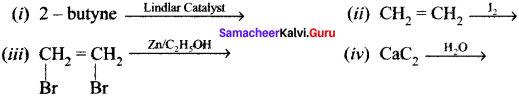
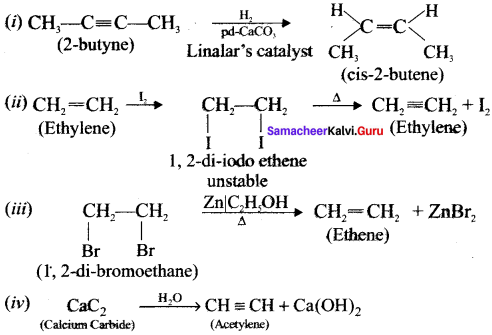

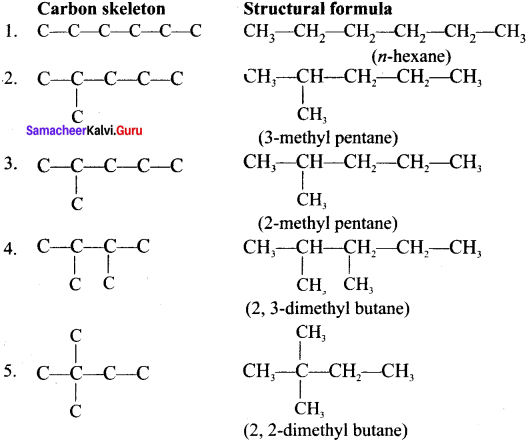
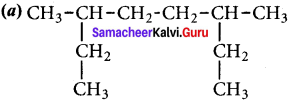

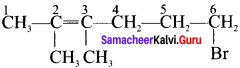
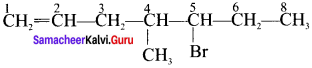
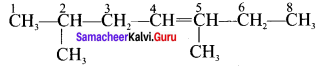
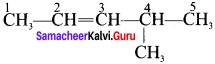

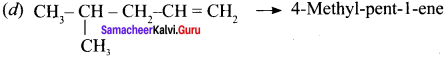
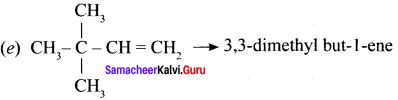
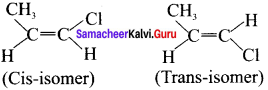


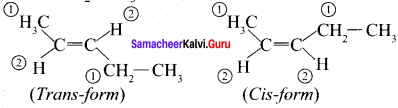
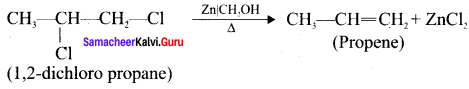
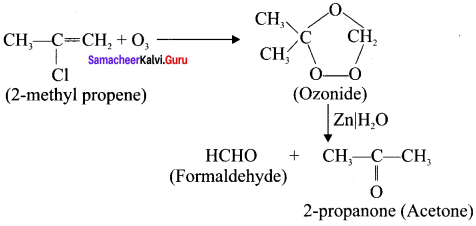
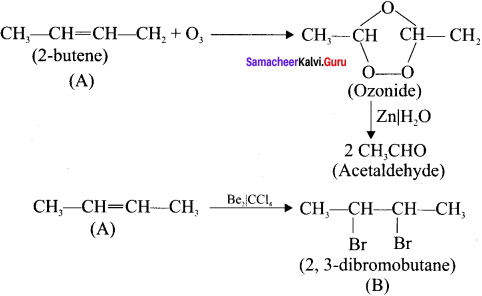
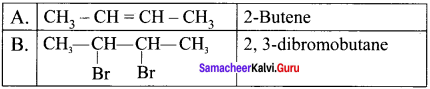

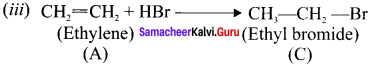
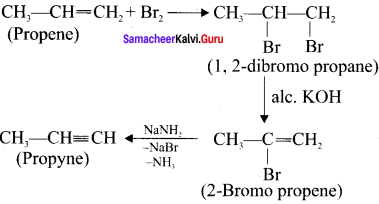
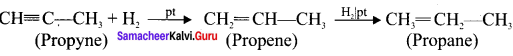
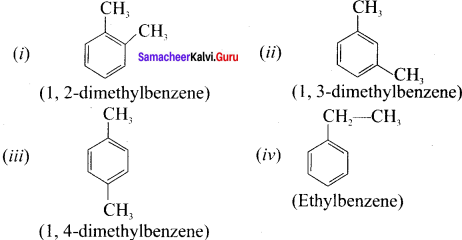
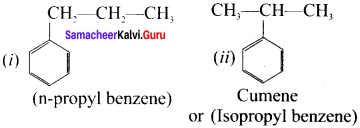

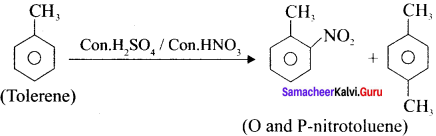

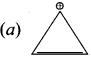
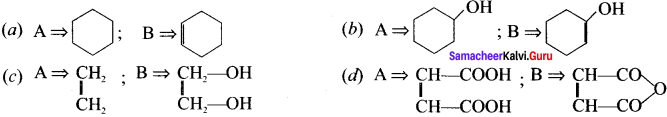
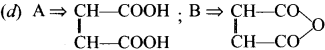





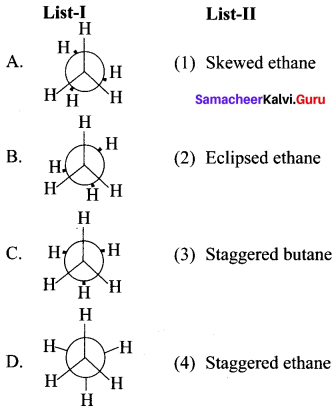





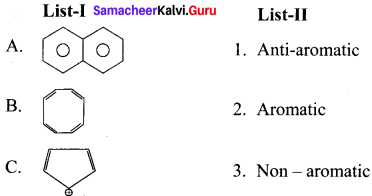


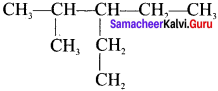
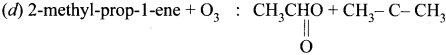
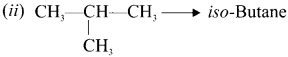

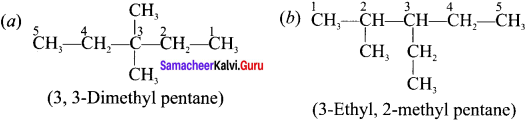
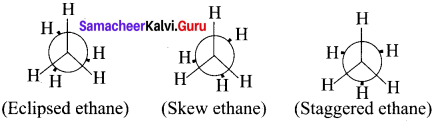
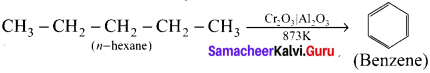
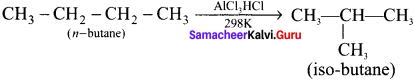
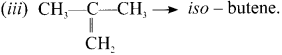

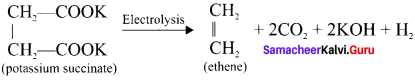
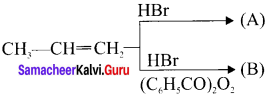
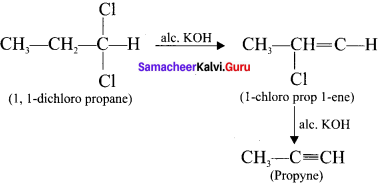
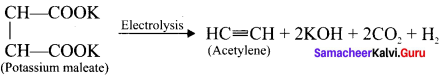
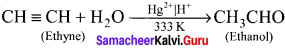
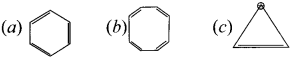

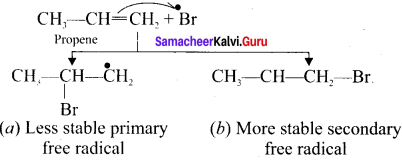
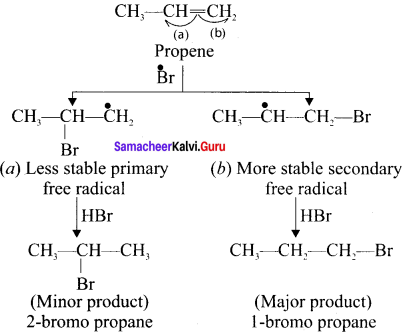
 Cyclopropyl cation.
Cyclopropyl cation.