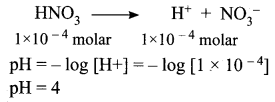
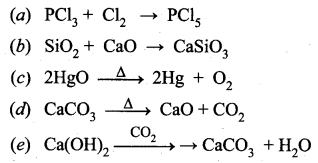
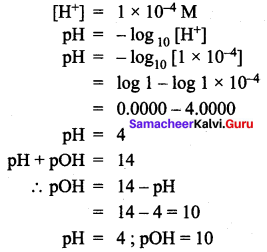
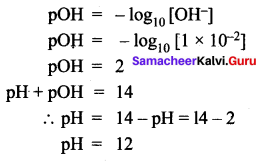
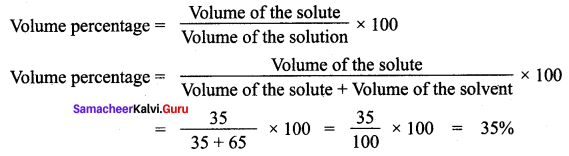
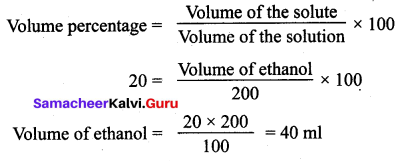
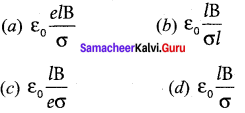
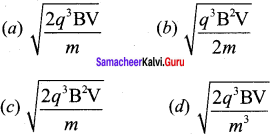
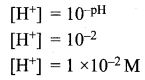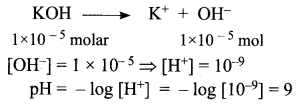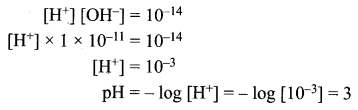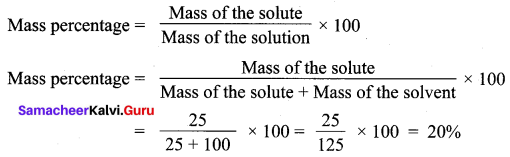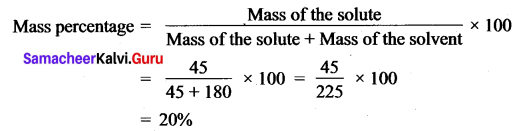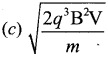You can Download Samacheer Kalvi 12th Maths Book Solutions Guide Pdf, Tamilnadu State Board help you to revise the complete Syllabus and score more marks in your examinations.
Tamilnadu Samacheer Kalvi 12th Maths Solutions Chapter 5 Two Dimensional Analytical Geometry – II Ex 5.2
12th Maths Exercise 5.2 Samacheer Kalvi Question 1.
Find the equation of the parabola in each of the cases given below:
(i) focus (4, 0) and directrix x = – 4.
(ii) passes through (2, -3) and symmetric about y-axis.
(iii) vertex (1, – 2) and focus (4, – 2).
(iv) end points of latus rectum(4, – 8) and (4, 8) .
Solution:
(i) Focus = F = (4, 0)
⇒ a = 4
Equation of directrix x = – 4
⇒ The curve open to the right. So the equation will be of the form y2 = 4 ax
Here a = 4
⇒ y2 = 4 (4) x (i.e.,)y2 = 16x
(ii) The parabola is symmetric about y axis. So the equation will be of the form
x2 = 4 ay
It passes through (2, -3)
⇒ 22 = 4a(-3)
4 = -12a ⇒ a = \(-\frac{1}{3}\) ⇒ 4a = \(-\frac{4}{3}\)
∴ Equation of parabola is x2 = \(-\frac{4}{3}\) y
3x2= – 4y.
(iii) The distance between vertex and focus = 3
(ie.,) a = 3
Parabola is open to the right.
So equation will be of the form y2 = 4ax
Here a = 3 ⇒ y2 = 12x
but the vertex is (1, -2)
So equation of the parabola is
(y + 2)2 = 12(x – 1)
(iv) Focus = (4, 0)
Equation of the parabola will be of the form .
y2 = 4ax
Here a = 4
⇒ y2 = 16x
12th Maths Exercise 5.2 Question 2.
ind the equation of the ellipse in each of the cases given below:
(i) foci (± 3, 0), e = \(\frac{1}{2}\)
(ii) foci (0, ± 4) and end points of major axis are(0, ± 5).
(iii) length of latus rectum 8, eccentricity = \(\frac{3}{5}\) and major axis on x -axis.
(iv) length of latus rectum 4 , distance between foci \(4 \sqrt{2}\) and major axis as y -axis.
Solution:
(i) Given ae = 3 and e = \(\frac{1}{2}\)
⇒ a(\(\frac{1}{2}\)) = 3 ⇒ a = 6
So a2 = 36
b2 = a2(1 – e2) = 36 (1 – \(\frac{1}{4}\)) = 36 × \(\frac{3}{4}\) = 27
Since Foci = (± 3, 0), major axis is along x-axis
So equation of ellipse is \(\frac{x^{2}}{36}+\frac{y^{2}}{27}\) = 1
(ii) From the diagram we see that major axis is along y-axis.
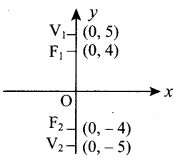
Also a = 5 and ae = 4
⇒ 5e = 4 ⇒ e = \(\frac{4}{5}\)
Now a = 5 ⇒ a2 = 25
ae = 4 ⇒ ae2 = 16
We know b2 = a2 (1 – e2) = a2 – a2e2 = 25 – 16 = 9
Equation of ellipse is \(\frac{x^{2}}{9}+\frac{y^{2}}{25}\) = 1
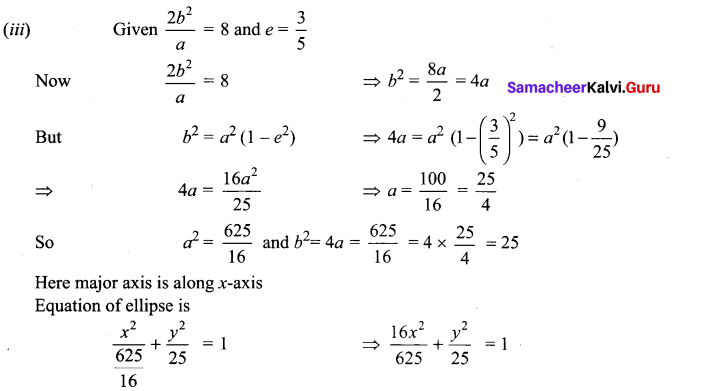
(iv) Given \(\frac{2 b^{2}}{a}\) = 4 and 2ae = \(4 \sqrt{2}\)
Now \(\frac{2 b^{2}}{a}\) = 4 2b2 = 4a
⇒ b2 = 2a
2ae = \(4 \sqrt{2}\) ae = \(2 \sqrt{2}\)
So a2e2 = 4(2) = 8
We know b2 = a2(1 – e2) = a2 – a2e2
⇒ 2a = a2 – 8 ⇒ a2 – 2a -8 = 0
⇒ (a – 4) (a +2) = 0 ⇒a = 4 or -2
As a cannot be negative
a = 4 So a2 = 16 and b2 = 2(4) = 8
Also major axis is along j-axis
So equation of ellipse is \(\frac{x^{2}}{8}+\frac{y^{2}}{16}\) = 1
12th Maths Exercise 5.2 8th Sum Question 3.
Find the equation of the hyperbola in each of the cases given below:
(i) foci (± 2, 0), eccentricity = \(\frac{3}{2}\)
(ii) Centre (2,1), one of the foci (8,1) and corresponding directrix x = 4 .
(iii) passing through(5, -2) and length of the transverse axis along JC axis and of length 8 units.
Solution:
(i) Given
ae = 2 and e = \(\frac{3}{2}\)
a( \(\frac{3}{2}\)) = 2 ⇒ a = \(\frac{4}{3}\) So a2 = \(\frac{16}{9}\)
b2 = a2(e2 – 1) = a2 e2 – a2 = 4 – \(\frac{16}{9}\) = \(\frac{20}{9}\)
Since the foci are (± 2, 0), transverse axis is along x-axis
So equation of hyperbola is
\(\frac{x^{2}}{16 / 9}-\frac{y^{2}}{20 / 9}\) = 1 ⇒ \(\frac{9 x^{2}}{16}-\frac{9 y^{2}}{20}\) = 1
(ii) Given Centre = (2, 1)
ae = 6 (distance between (2, 1) and (8, 1)) ……………. (1)
Also \(\frac{a}{e}\) = 2 ⇒ a = 2e
Equation of directrix is x = 4 [(i.e.,) (x – 2 = 2) Since centre is (2, 1)]
⇒ \(\frac{a}{e}\) = 2
Given ae = 6 ⇒ a2 e2 = 36
(i.e.) (2e)2 (e)2 = 36
⇒ 4e4 = 36 ⇒ e4 = 9
⇒ e = \(\sqrt{3}\)
Now e = \(\sqrt{3}\) a = \(2\sqrt{3}\)
∴ a2 = 4 × 3 = 12
b2 = a2 (e2 – 1) = a2 e2 – a2 = 36 – 12 = 24
So here Centre = (2, 1)
So equation of hyperbola is
\(\frac{(x-2)^{2}}{12}-\frac{(y-1)^{2}}{24}\) = 1
(iii) Length of the transverse axis = 8
2a = 8 ⇒ a = 4
Transverse axis is along x-axis
So of equation of hyperbola is will be
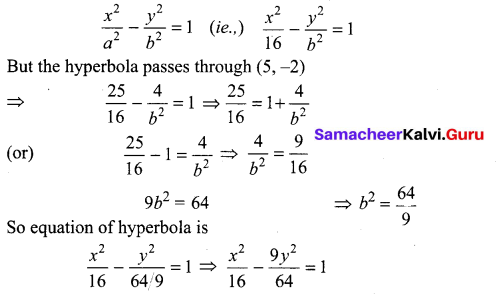
12th Maths 5th Chapter Samacheer Kalvi Question 4.
Find the vertex, focus, equation of directrix and length of the latus rectum of the following:
(i) y2 = 16x
(ii) x2 = 24y
(iii) y2 = -8x
(iv) x2 – 2x + 8y + 17 = 0
(v) y2 – 4y – 8x + 12 = 0
Solution:
(i) y2 = 16x
It is of the form y2 = 4ax (type I)
Here 4a = 16 ⇒ a = 4
Vertex = (0, 0)
Focus = (a, 0) = (4, 0)
Equation of directrix x + 4 = 0 (or) x = – 4
Length of latus rectum = 4a = 16 .
(ii) x2 = 24y
This is of the form x2 = 4ay (type III)
4a = 24 ⇒ a = 6
Vertex = (0, 0)
Focus = (0, a) = (0, 6)
Equation of directrix is y + a= 0 (i.e.,) y + 6 = 0 (or) y = -6
Length of latus rectum = 4a = 24.
(iii) y2 = -8x
This is of the form y2 = – 4ax (type II)
Here 4a = 8 ⇒ a = 2
Vertex = (0, 0)
Focus = (- a, 0) = (-2, 0)
Equation of directrix is x – 2 = 0 (or) x = 2
Length of latus rectum = 4a = 8.
(iv) x2 – 2x + 8y + 17 = 0
x2 – 2x = -8y – 17
x2 – 2x + 1 – 1 = – 8y — 17
(x – 1)2 = – 8y – 17 + 1 = – 8y + 16
(x – 1)2 = – 8 (y – 2)
Taking x – 1 = X and y – 2 = Y.
We get X2 = – 8Y.
This is of the form x2 = – 4ay (type IV)
Where 4a = 8 ⇒ a = 2
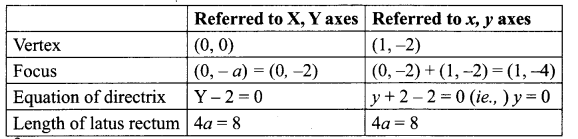
(v) y2 – 4y = 8x – 12 = 0
y2 – 4y + 4 = 8x – 12 + 4
(y – 2)2 = 8x – 8 = 8 (x – 1)
Taking x – 1 = X and y – 2 = Y.
We get Y2 = 8X.
This is of the form y2 = 4ax (type IV)
Where 4a = 8 ⇒ a = 2

12th Maths 5.2 Exercise Question 5.
Identify the type of conic and find centre, foci, vertices, and directrices of each of the following:
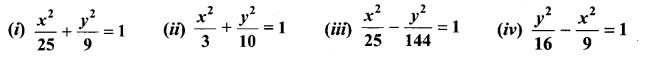
Solution:
(i) \(\frac{x^{2}}{25}+\frac{y^{2}}{9}\) = 1
It is of the form \(\frac{x^{2}}{a^{2}}+\frac{y^{2}}{b^{2}}\) = 1, which is an ellipse
Here a2 = 25, b2 = 9
a = 5, b = 3
e2 = \(\frac{a^{2}-b^{2}}{a^{2}}=\frac{25-9}{25}=\frac{16}{25}\) ⇒ e = \(\frac{4}{5}\)
Now e = \(\frac{4}{5}\) and a = 5 ⇒ ae = 4 and \(\frac{a}{e}=\frac{5}{4 / 5}=\frac{25}{4}\)
Here the major axis is along x axis
∴ Centre = (0, 0)
Foci = (± ae, 0) = (± 4, 0)
Vertices = (± a, 0) = (±5, 0)
Equation of directrix x = ± \(\frac{a}{e}\) (ie.,) x = ± \(\frac{25}{4}\)
(ii) \(\frac{x^{2}}{3}+\frac{y^{2}}{10}\) = 1
It is an ellipse and here (always a >b)
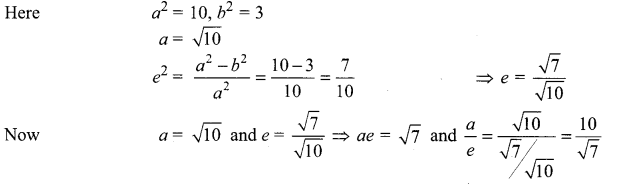
Here the major axis is alongy-axis
Centre = (0, 0)
Foci = (0, ± ae) = (0, ± \(\sqrt{7}\))
Vertices = (0, ± a) = (0, ± \(\sqrt{10}\) )
Equation of directrices y = ± \(\frac{10}{\sqrt{7}}\)
(iii) \(\frac{x^{2}}{25}-\frac{y^{2}}{144}\) = 1
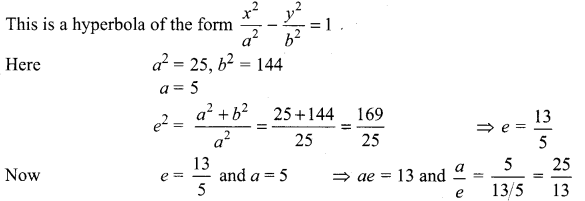
Now, Here transverse axis is along x-axis
Centre = (0, 0)
Vertices = (± a, 0) = (± 5, 0)
Foci = (± ae, 0) = (± 13, 0)
Equation of directrices x = ± \(\frac{a}{e}\) (ie.,) x = ± \(\frac{25}{13}\)
(iv) \(\frac{y^{2}}{16}-\frac{x^{2}}{9}\) = 1
It is a hyperbola. Here transverse axis is along y-axis
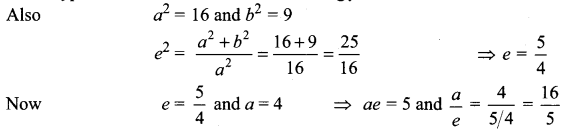
Now Centre = (0, 0)
Vertices = (0, ± a) = (0, ± 4)
Foci = (0, ± ae) = (0, ± 5)
Equation of directrices y = ± \(\frac{16}{5}\)
Class 12 Maths Chapter 5 Exercise 5.2 Question 6.
Prove that the length of the latus rectum of the hyperbola \(\frac{x^{2}}{a^{2}}-\frac{y^{2}}{b^{2}}\) = 1 is \(\frac{2 b^{2}}{a}\)
Solution:
The latus rectum LL’ of the hyperbola \(\frac{x^{2}}{a^{2}}-\frac{y^{2}}{b^{2}}\) = 1 passes through the focus(ae, 0)
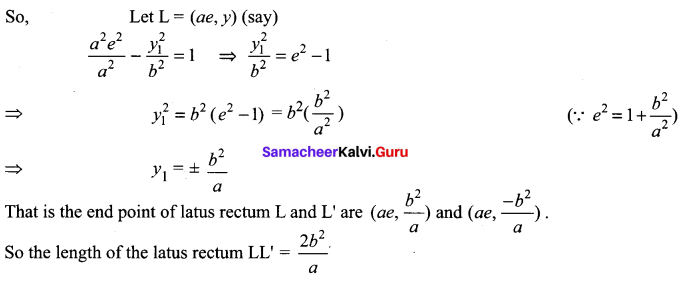
12th Maths 5.2 Question 7.
Show that the absolute value of the difference of the focal distances of any point P on the hyperbola is the length of its transverse axis.
Solution:
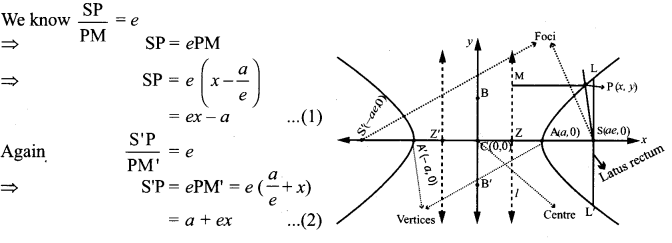
∴ S’P – SP = (a + ex) – (ex – a)
a + ex – ex + a = 2a (transverse axis)
Question 8.
Identify the type of conic and find centre, foci, vertices, and directrices of each of the following:
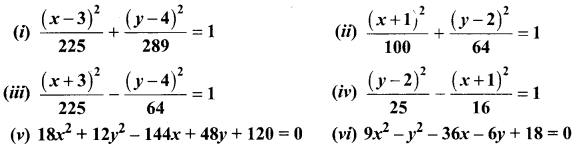
Solution:
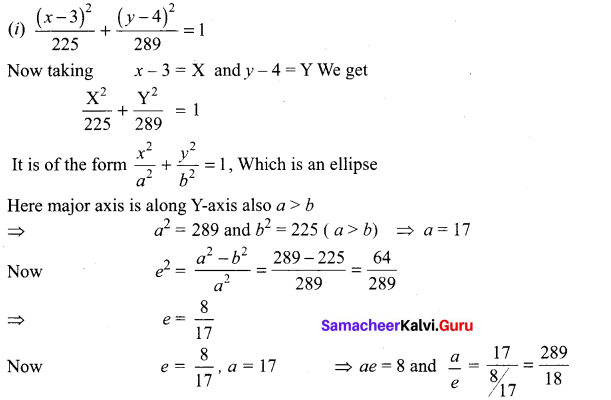
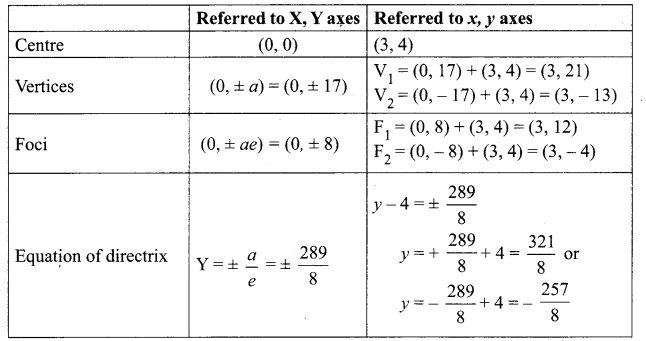

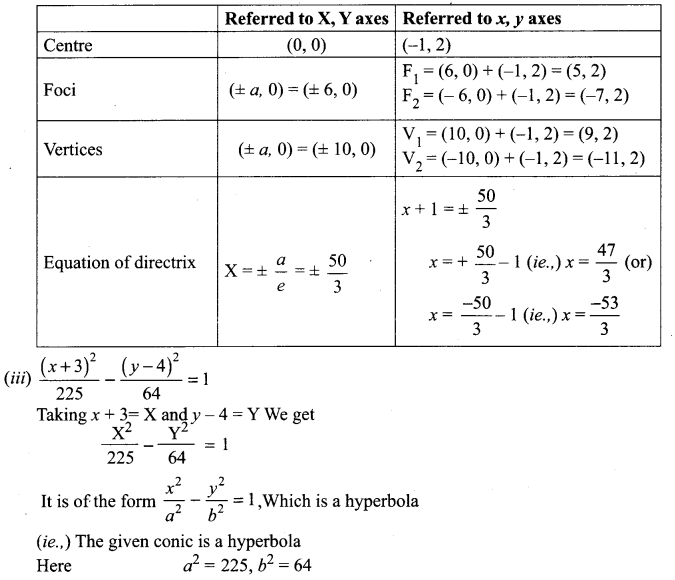
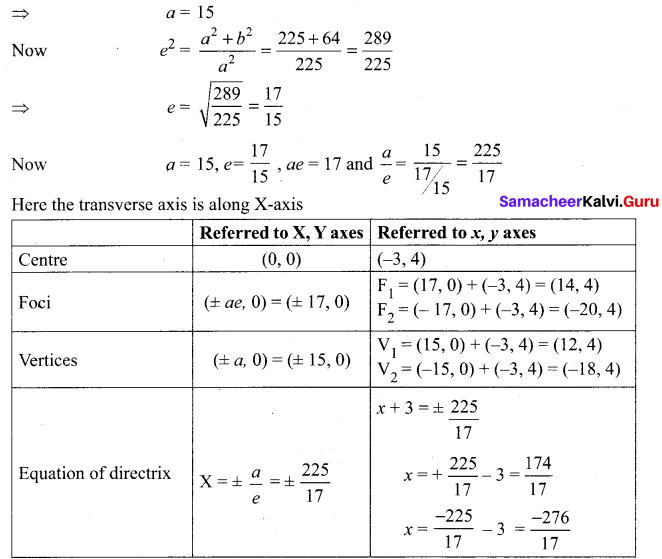
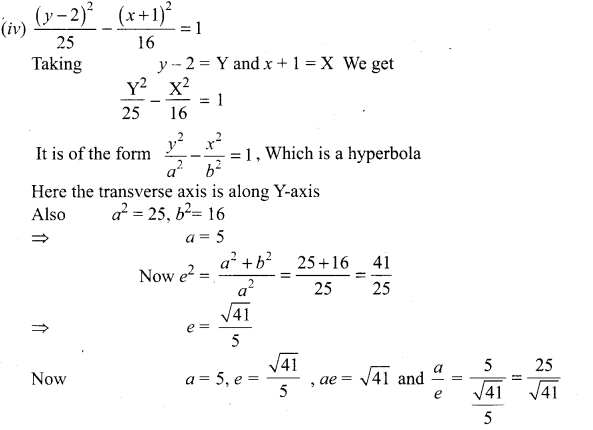
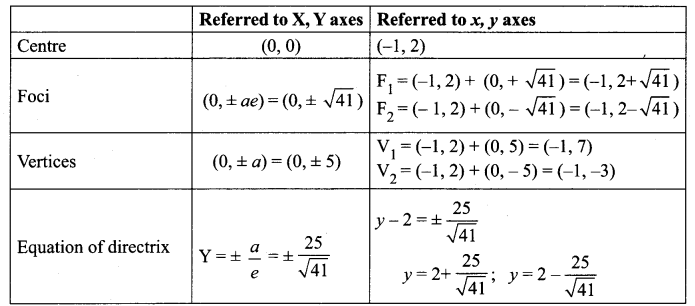
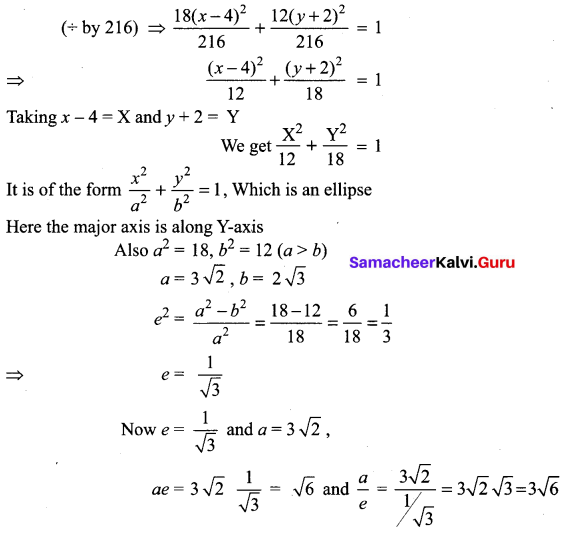
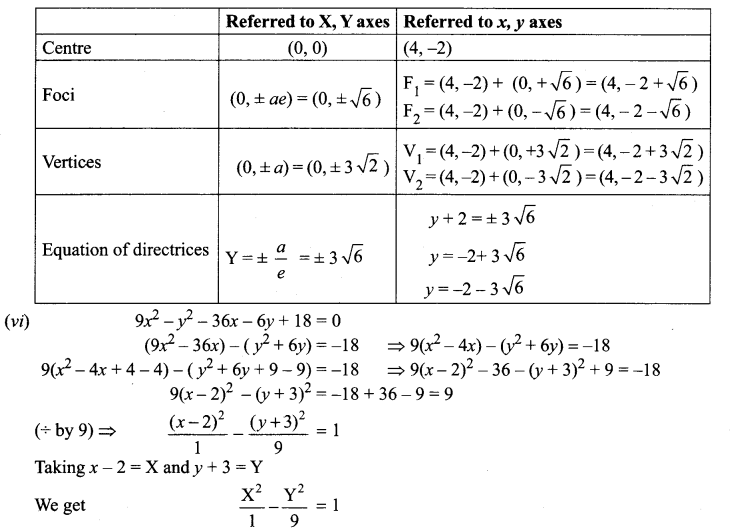
(v) 18x2 + 12y2 – 144x + 48y + 120 = 0
(18x2 – 144x) + (12y2 + 48y) = -120
18(x2 – 8x) + 12 (y2 + 4y) = -120
18(x2 – 8x + 16 – 16) + 12(y2 + 4y + 4 – 4) = -120
18(x – 4)2 – 288 + 12(y + 2)2 – 48 = – 120
18(x – 4)2 + 12(y + 2)2 = -120 + 288 + 48 = 216
Samacheer Kalvi 12th Maths Solutions Chapter 5 Two Dimensional Analytical Geometry – II Ex 5.2 Additional Problems
Question 1.
Find the equation of the ellipse if centre is (3, – 4), one of the foci is (3 + \(\sqrt{3}\), – 4) and e = \(\frac{\sqrt{3}}{2}\)
Solution:
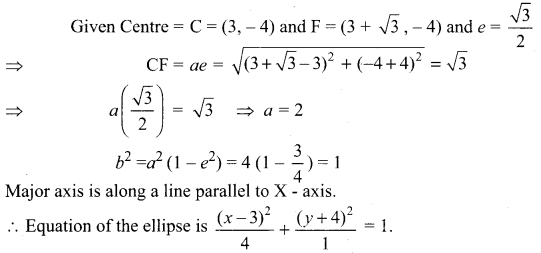
Question 2.
Find the equation of the hyperbola if centre (1, -2); length of the transverse axis is 8; e = \(\frac{5}{4}\) and the transverse axis is parallel to X-axis.
Solution:
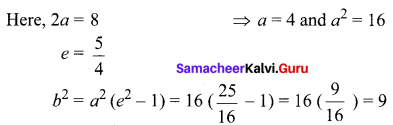
Here, centre = (1, -2) and transverse axis is parallel to X-axis.

12th Maths Chapter 5 Exercise 5.2 Question 3.
Find axis, vertex, focus and equation of directrix for y2 + 8x – 6y + 1 = 0
Solution:
y2 – 6y = – 8x – 1
y2 – 6y + 9 = – 8x – 1 + 9
(y – 3)2 = – 8x + 8 = – 8(x – 1)
Comparing this equation with Y2 = – 4aX we get
4a = 8 or a = 2
Vertex is (0, 0)
x – 1 = 0 ⇒ x = 1, y – 3 = 0 ⇒ y = 3
Axis y – 3 = 0, Vertex = (1, 3)
Focus is (- a, 0) = (-2, 0) + (1, 3) = (-1, 3)
Equation of directrix is x – a = 0. i.e., X – 2 = 0
⇒ x – 1 – 2 = 0 ⇒ x – 3 = 0
Latus rectum x + a = 0 i.e., x – 1 + 2 = 0
x + 1 = 0
Length of latus rectum = 4a = 8
Question 4.
Find axis, Vertex focus and equation of directrix for x2 – 6x – 12y – 3 = 0.
Solution:
x2 – 6x = 12y + 3
x2 – 6x + 9 = 12y + 3 + 9 = 12y + 12
(x – 3)2 = 12(y + 1)
This equation is of the form X2 = 4aY
4a = 12
⇒ a = 3
Vertex is x – 3 = 0 ; y + 1 = 0
⇒ x = 3 ; y = -1
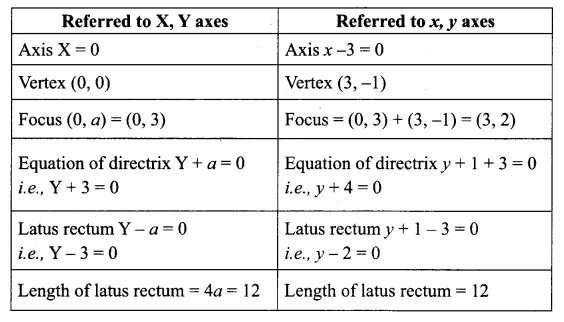
Question 5.
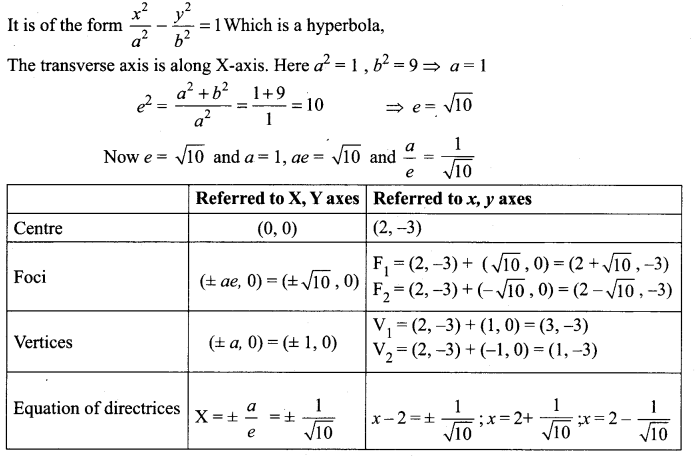
Find the eccentricity, centre, foci and vertices of the following hyperbolas: x2 – 4y2 – 8x – 6y – 18 = 0
Solution:
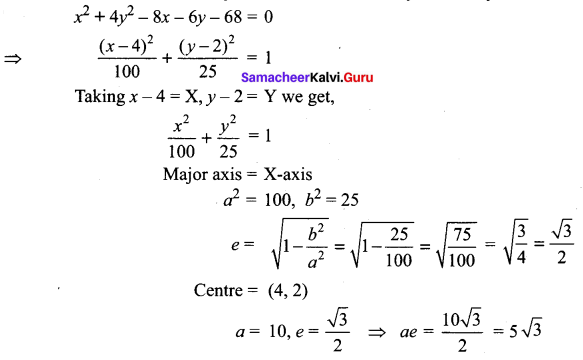
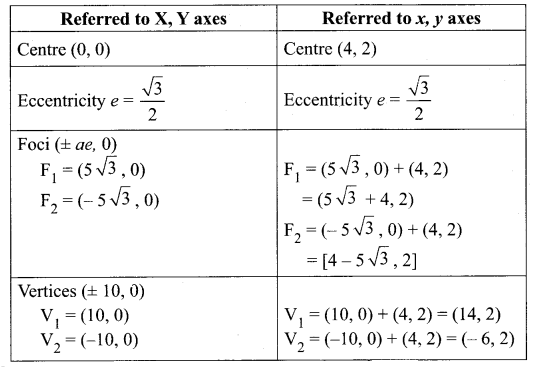
Question 6.
Find the eccentricity, centre, foci, vertices of 9x2 + 4y2 = 36
Solution:
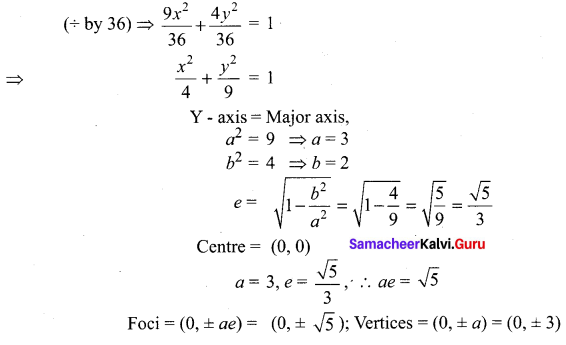
Ex 5.2 Class 12 Question 7.
Find the eccentricity, centre, foci and vertices of the following hyperbolas:
x2 – 4y2 + 6x + 16y – 11 = 0
Solution:
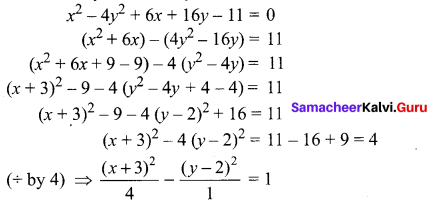
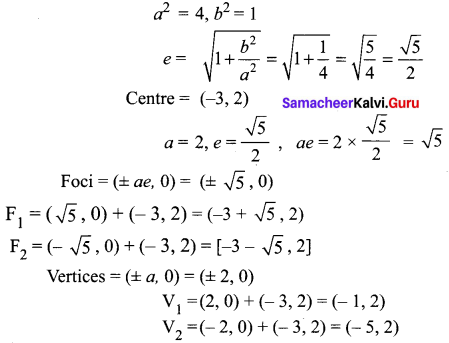
10th Maths Exercise 5.2 Samacheer Kalvi Question 8.
Find the eccentricity, centre, foci and vertices of the following hyperbolas:
x2 – 3y2 + 6x + 6y + 18 = 0
Solution: