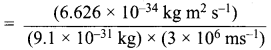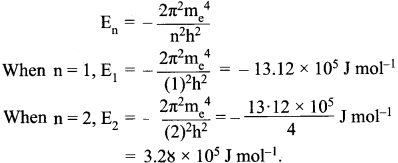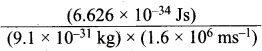You can Download Samacheer Kalvi 11th Maths Book Solutions Guide Pdf, Tamilnadu State Board help you to revise the complete Syllabus and score more marks in your examinations.
Tamilnadu Samacheer Kalvi 11th Maths Solutions Chapter 3 Trigonometry Ex 3.4
11th Maths Exercise 3.4 Samacheer Kalvi Question 1.
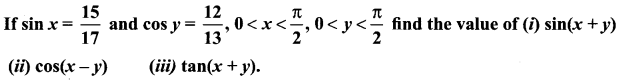
Solution:
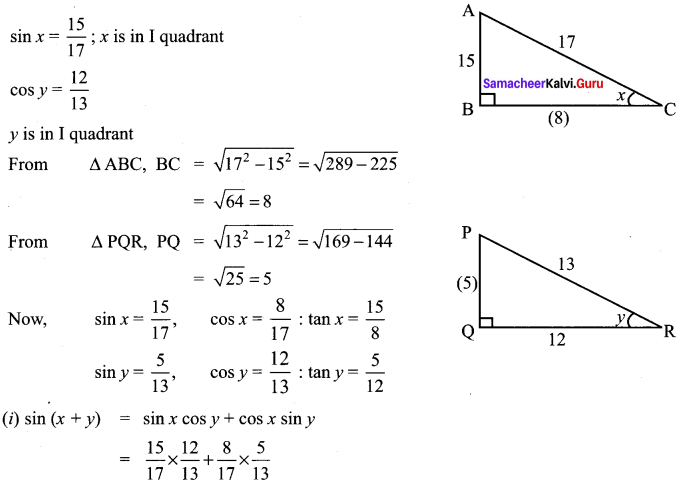

11th Maths Exercise 3.4 Question 2.
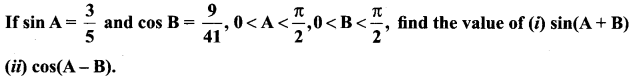
Solution:
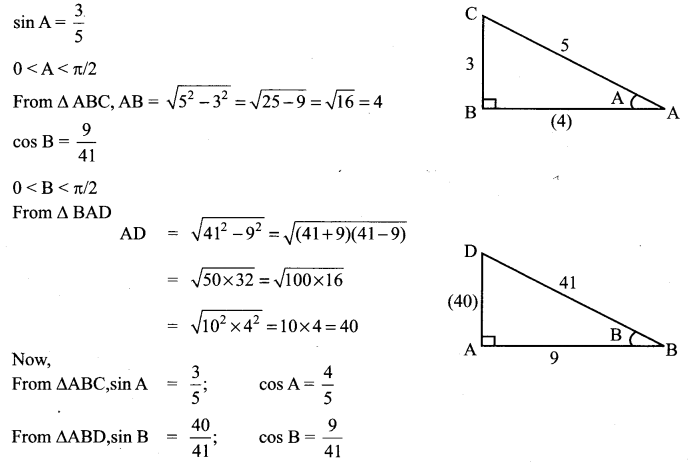
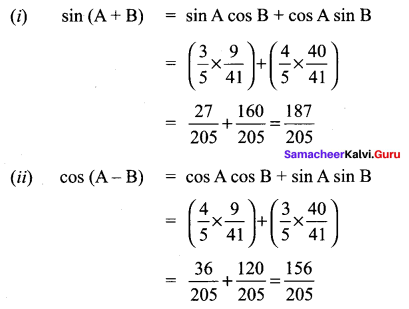
11th Maths Exercise 3.4 Answers Question 3.
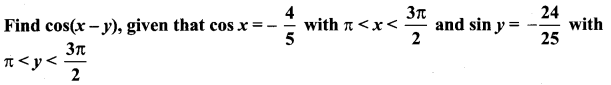
Solution:

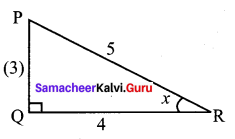
since x is in III quadrant
Both sin x and cos x are negative
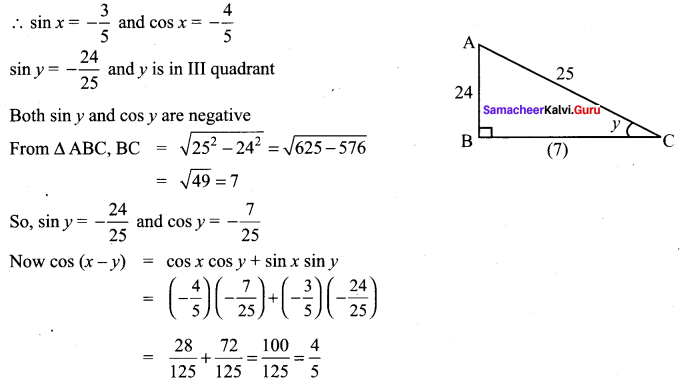
11 Maths Samacheer Solution Question 4.
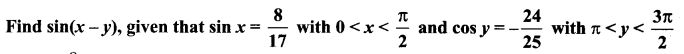
Solution:
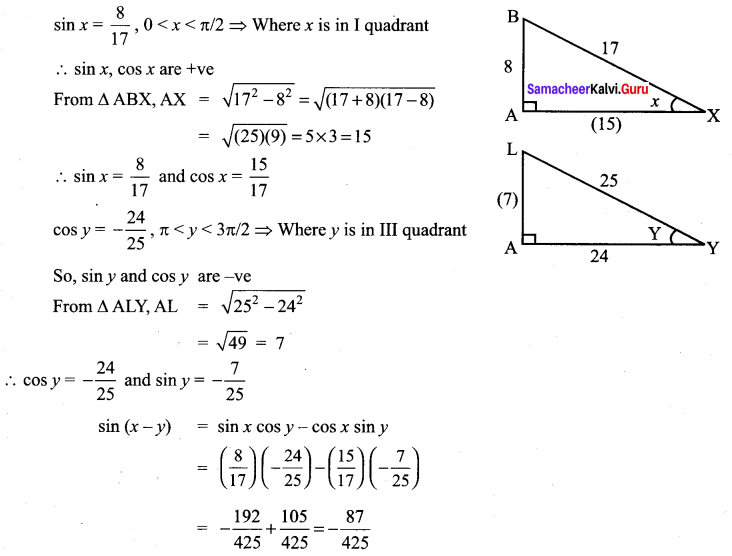
Class 11 Maths Ex 3.4 Solutions Question 5.
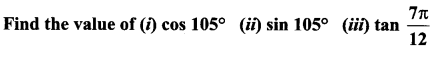
Solution:
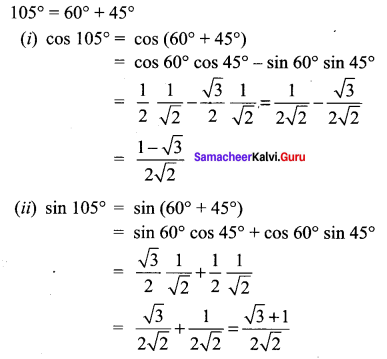
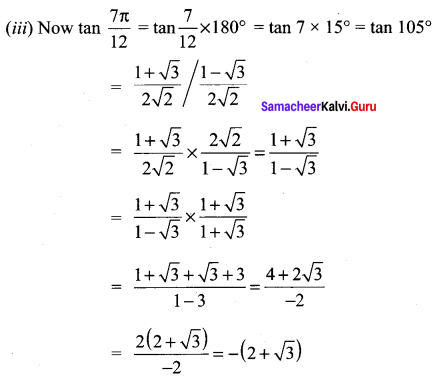
Samacheer Kalvi 11 Maths Solutions Question 6.
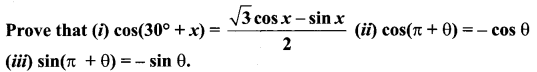
Solution:
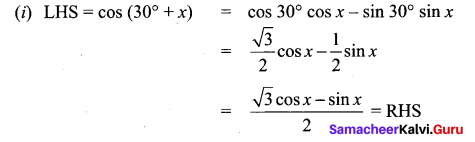
(ii) L.HS = cos(π + θ) = cos(180° + θ)
= cos 180° cos θ – sin 180° sin θ
= (-1) cos θ – (0) sin θ
= – cos θ = RHS
(iii) LHS = sin (π + θ) = sin π cos θ + cos π sin θ
= (0) cos θ + (-1) sin θ
= -sin θ = RHS
Samacheer Kalvi Guru 11th Maths Solution Question 7.
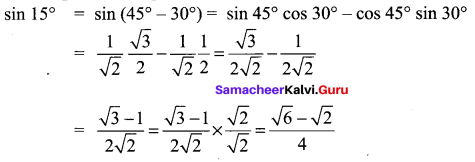
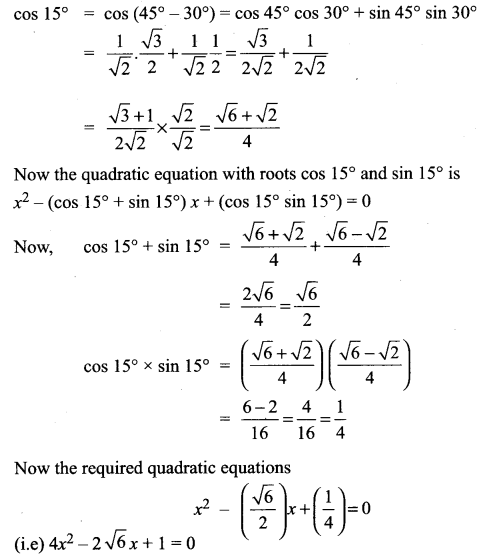
Find a quadratic equation whose roots are sin 15° and cos 15°
Solution:
11th Maths Samacheer Solutions Question 8.
Expand cos(A + B + C). Hence prove that cos A cos B cos C = sin A sin B cos C + sin B sin C cos A + sin C sin A cos B, if A + B + C = \(\frac{\pi}{2}\)
Solution:
Taking A + B = X and C = Y
We get cos (X + Y) = cos X cos Y – sin X sin Y
(i.e) cos (A + B + C) = cos (A + B) cos C – sin (A + B) sin C
= (cos A cos B – sin A sin B) cos C – [sin A cos B + cos A sin B] sin C
cos (A + B + C) = cos A cos B cos C – sin A sin B cos C – sin A cos B sin C – cos A sin B sin C If (A + B + C) = π/2 then cos (A + B + C) = 0
⇒ cos A cos B cos C – sin A sin B cos C – sin A cos B sin C – cos A sin B sin C = 0
⇒ cos A cos B cos C = sin A sin B cos C + sin B sin C cos A + sin C sin A cos B
Trigonometry 3.4 Class 11 Question 9.
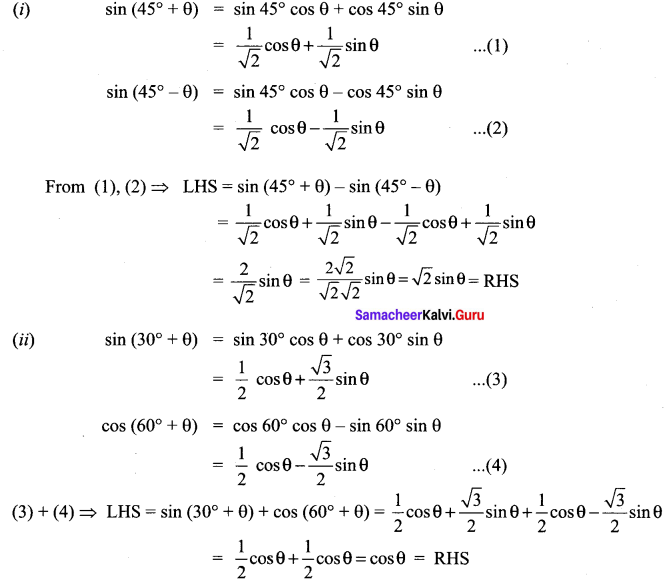
Prove that
(i) sin(45° + θ) – sin(45° – θ) = \(\sqrt{2}\)sin θ.
(ii) sin(30° + θ) + cos(60° + θ) = cos θ.
Solution:
Samacheer Kalvi Guru 11th Maths Question 10.
If a cos(x + y) = b cos(x – y), show that (a + b) tan x = (a – b) cot y.
Solution:
a cos (x + y) = b cos (x – y)
a[cos x cos y – sin x sin y] = 6[cos x cos y + sin x sin y]
(i.e) a cos x cos y – a sin x sin y = b cos x cos y + b sin x sin y
a cos x cos y – b sin x sin y = a sin x sin y + b cos x cos y
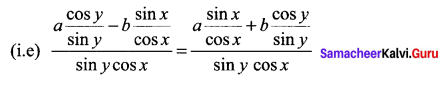
⇒ a cot y – b tan x = a tan x + b cot y
a cot y – b cot y = a tan x + b tan x
⇒ (a + b) tan x = (a – b) cot y.
Samacheer Kalvi Class 11 Maths Solutions Question 11.
Prove that sin 105° + cos 105° = cos 45°.
Solution:
sin 105° = sin (60°+ 45°)
= sin 60° cos 45° + cos 60° cos 45°
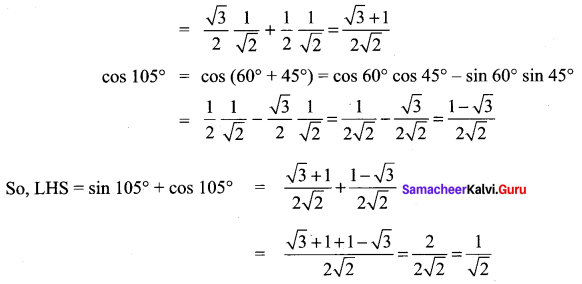
= cos 45° = RHS
Samacheer Kalvi Maths 11th Question 12.
Prove that sin 75° – sin 15° = cos 105° + cos 15°.
Solution:
RHS = cos 105° + cos 15° = cos (90° + 15°) + cos (90° – 75°)
= – sin 15° + sin 75°
= sin 75° – sin 15° = LHS
Samacheer Kalvi 11th Maths Solutions Question 13.
Show that tan 75° + cot 75° = 4
Solution:
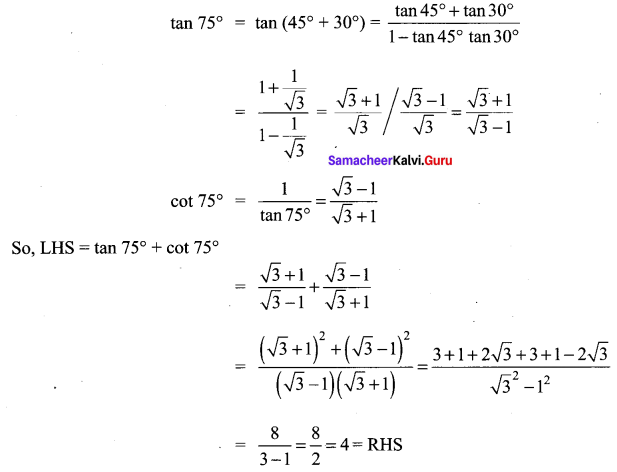
Samacheer Kalvi Guru 11 Maths Question 14.
Prove that cos(A + B) cos C – cos(B + C) cos A = sin B sin(C – A).
Solution:
LHS = (cos A cos B – sin A sin B) cos C – (cos B cos C – sin B sin C) cos A
= cos A cos B cos C – sin A sin B cos C – cos A cos B cos C + cos A sin B sin C
= cos A sin B sin C – sin A sin B cos C
= sin B [sin C cos A – cos C sin A]
= sin B [sin (C – A)] = RHS
Samacheerkalvi.Guru 11th Maths Question 15.
Prove that sin(n + 1) θ sin(n – 1) θ + cos(n + 1) θ cos(n – 1)θ = cos 2θ, n ∈ Z.
Solution:
Taking (n + 1) θ = A and (n – 1) θ = B
LHS = sin A sin B + cos A cos B
= cos (A – B)
= cos[(n + 1) – (n – 1)]θ
= cos (n + 1 – n + 1)θ = cos 2θ = RHS
Samacheer Kalvi.Guru 11th Maths Question 16.
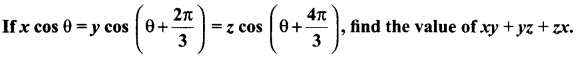
Solution:
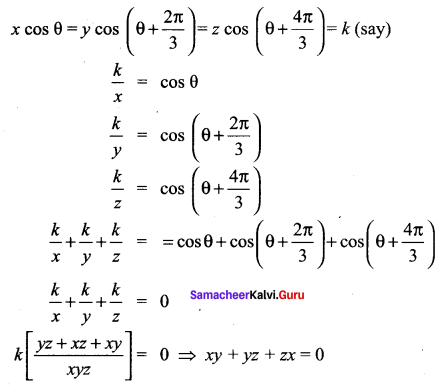
Samacheer Kalvi 11th Maths Question 17.
Prove that
(i) sin(A + B) sin(A – B) = sin2 A – sin2 B
(ii) cos(A + B) cos(A – B) = cos2 A – sin2 B = cos2 B – sin2 A
(iii) sin2(A + B) – sin2(A – B) = sin2A sin2B
(iv) cos 8θ cos 2θ = cos2 5θ – sin2 3θ
Solution:
(i) LHS = sin (A + B) sin (A – B)
= (sin A cos B + cos A sin B) (sin A cos B – cos A sin B)
= sin2A cos2 B – cos2 A sin2 B
= sin2 A (1 – sin2 B) – (1 – sin2 A) sin2 B
= sin2 A – sin2 A sin2 B – sin2 B + sin2 A sin2 B
= sin2 A – sin2 B = RHS
(ii) LHS = cos (A + B) cos (A – B) = (cos A cos B – sin A sin B) (cos A cos B + sin (A sin B)
= cos2 A cos2 B – sin2 A sin2 B
= cos2 A (1 – sin2 B) – (1 – cos2 A) sin2 B
= cos2 A – cos2 A sin2 B – sin2 B + cos2 A sin2 B
= cos2 A – sin2 B = RHS
Now cos2 A – sin2 B = (1 – sin2 A) – (1 – cos2 B)
= 1 – sin2 A – 1 + cos2 B
= cos2 B – sin2 A
(iii) sin2 A – sin2 B = sin (A + B) sin (A – B)
LHS = sin2 (A + B) – sin2 (A – B) = sin [(A + B) + (A – B)] [sin (A + B) – (A – B)]
= sin 2A sin 2B = RHS
(iv) LHS = cos 8θ cos 2θ
= cos (5θ + 3θ) cos (5θ – 3θ) .
We know cos (A + B) cos (A – B) = cos2 A – sin2 B
∴ cos (5θ + 3θ) cos (5θ – 3θ) = cos2 5θ – sin2 3θ = RHS
Samacheer Kalvi 11th Maths Book Solutions Question 18.
Show that cos2 A + cos2 B – 2 cos A cos B cos(A + B) = sin2(A + B).
Solution:
LHS = cos2 A + cos2 B – 2 cos A cos B [cos A cos B – sin A sin B]
= cos2 A + cos2 B – 2 cos2 A cos2 B + 2 sin A cos A sin B cos B
= (cos2 A – cos2 A cos2 B) + (cos2 B – cos2 A cos2 B) + 2 sin A cos A sin B cos B
= cos2 A (1 – cos2 B) + cos2 B (1 – cos2 A) + 2 sin A cos A sin B cos B
= cos2 A sin2 B + cos2 B sin2 A + 2 sin A cos B sin B cos A
= (sin A cos B + cos A sin B)2
= sin2 (A + B) = RHS
Samacheer Kalvi 11th Maths Solution Question 19.
If cos(α – β) + cos(β – γ) + cos(γ – α) = \(-\frac{3}{2}\), then prove that cos α + cos β + cos γ = sin α + sin β + sin γ
Solution:
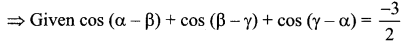
2 cos (α – β) + 2cos (β – γ) + 2cos (γ – α) = -3
2cos(α – β) + 2cos(β – γ) + 2cos (γ – α) + 3 = 0
[2 cos α cos β + 2 sin α sin β] + [2 cos β cos γ + 2 sin β sin γ] + [2 cos γ cos α + sin γ sin α] + 3 = 0
= [2 cos α cos β + 2 cos β cos γ + 2 cos γ cos α] + [2 sin α sin β + 2 sin β sin γ + 2 sin γ sin α] + (sin2 α + cos2 α) + (sin2 β + cos2 β) + (sin2 γ + cos2 γ) = 0
⇒ (cos2 α + cos2 β + cos2 γ + 2 cos α cos β + 2 cos β cos γ + 2 cos γ cos α) + (sin2 α + sin2 β) + (sin2 γ + 2 sin α sin β + 2 sin β sin γ + 2 sin γ sin α) = 0
(cos α + cos β + cos γ)2 + (sin α + sin β + sin γ)2 = 0
=(cos α + cos β + cos γ) = 0 and sin α + sin β + sin γ = 0
Hence proved
Samacheer 11 Maths Solutions Question 20.
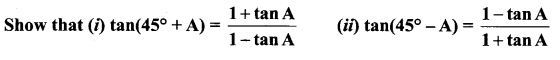
Solution:
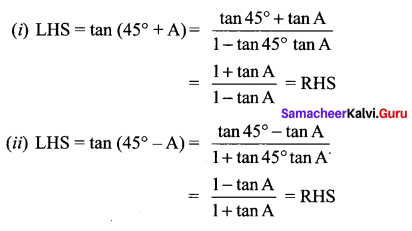
Samacheer Kalvi 11th Maths Book Question 21.
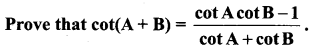
Solution:
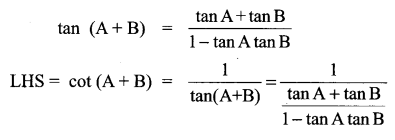
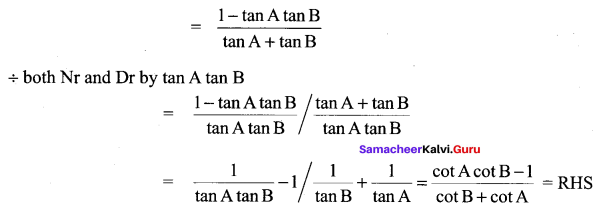
11th Maths Solutions Samacheer Kalvi Question 22.
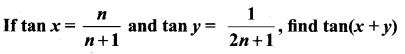
Solution:
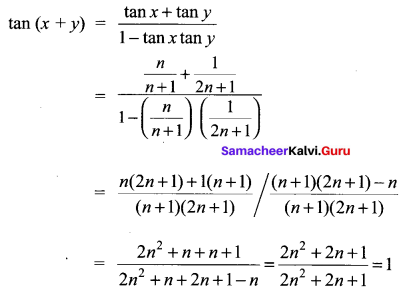
11th Samacheer Kalvi Maths Question 23.
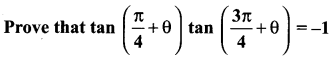
Solution:
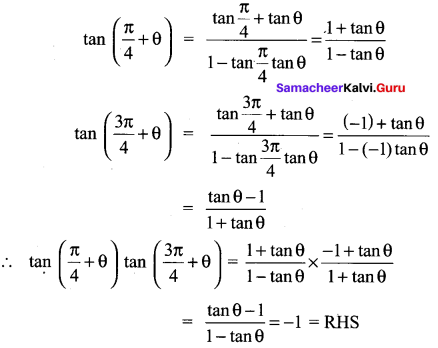
Question 24.
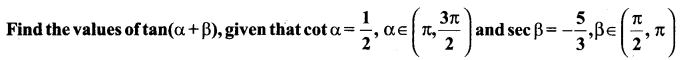
Solution:
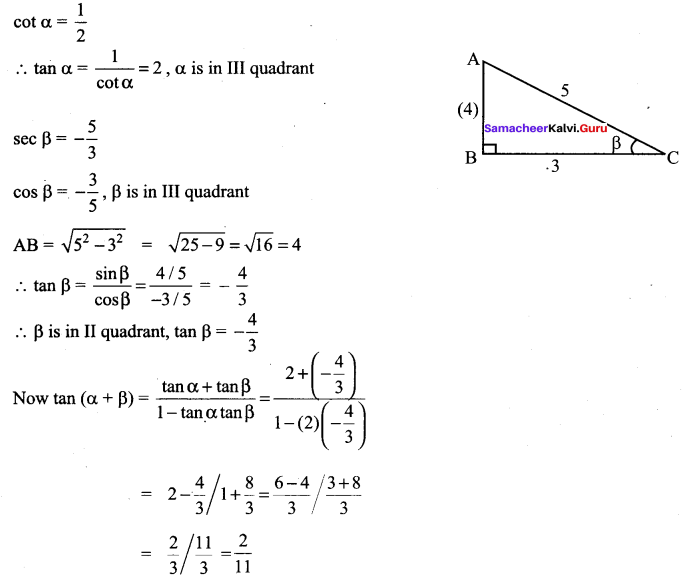
Maths Samacheer Kalvi 11th Question 25.

Solution:
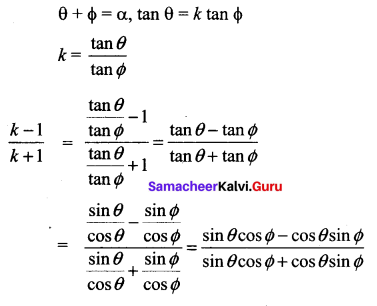
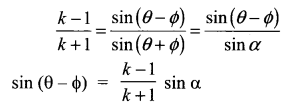
Samacheer Kalvi 11th Maths Solutions Chapter 3 Trigonometry Ex 3.4 Additinal Questions
10th Maths Exercise 3.4 Samacheer Kalvi Question 1.
Prove that sin (A + B) sin (A – B) = cos2 B – cos2 A
Solution:
LHS = sin (A + B) sin (A – B)
= (sin A cos B + cos A sin B) (sin A cos B – cos A sin B)
= sin2 A cos2 B – cos2 A sin2 B
= (1 – cos2 A) cos2 B – cos2 A (1 – cos2 B)
= cos2 B – cos2 A cos2 B – cos2 A + cos2 A cos2 B
= cos2 B – cos2 A = RHS
Question 2.
Prove that
(i) sin A + sin(120° + A) + sin (240° + A) = 0
(ii) cos A + cos (120° +A) + cos (120° – A) = 0
Solution:
(i) sin A + sin(120° + A) + sin (240° + A)
= sin A+ sin 120° cos A + cos 120° sin A + sin 240° cos A + cos 240° sin A …… (1)
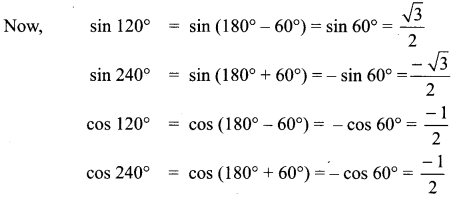
By substituting these values in (1), we get,
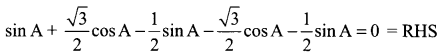
(ii) cos 120° = cos (180° – 60°) = – cos 60° = \(\frac{-1}{2}\)
LHS = cos A + cos (120° + A) + cos (120° – A)
= cos A + cos 120° cos A – sin 120° sin A + cos 120° cos A + sin 120° sin A
= cos A + 2 cos 120° cos A
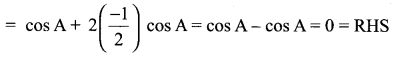
Question 3.
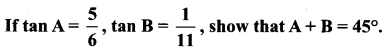
Solution:
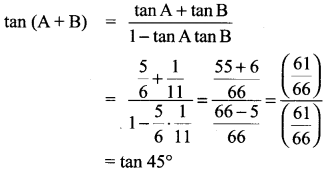
tan (A + B) = 1 ⇒ A+ B = 45°
Question 4.
If A + B = 45°, show that (1 + tan A) (1 + tan B) = 2 and hence deduce the value of tan \(22 \frac{1^{0}}{2}\).
Solution:
Given A + B = 45° ⇒ tan (A + B) = tan 45°
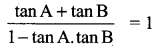
(i.e.) tan A + tan B = 1 – tan A.tan B
(i.e.) 1 + tan A + tan B = 2 – tan A tan B (add 1 on both sides)
1 + tan A + tan B + tan A tan B = 2
(i.e.) (1 + tan A) (1 + tan B) = 2
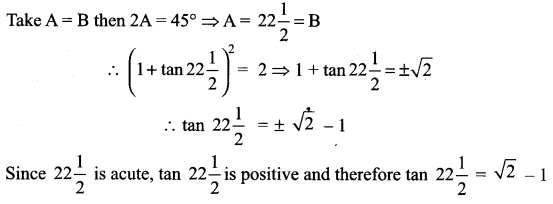
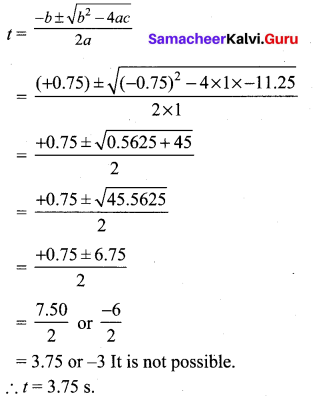
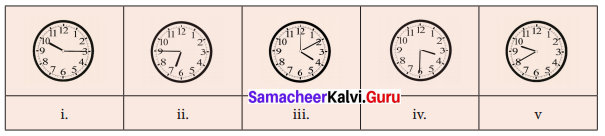
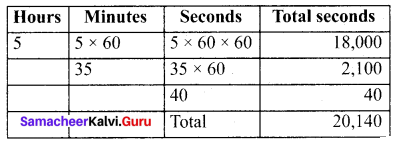

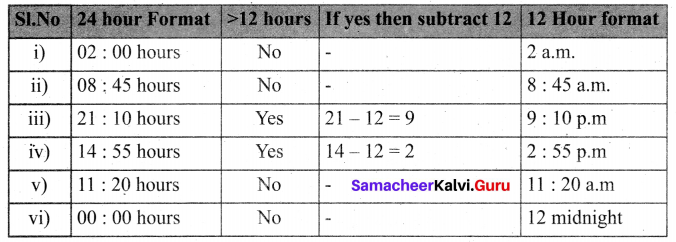






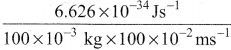
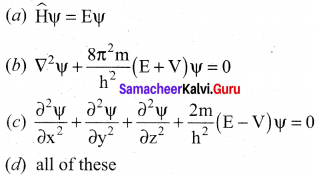

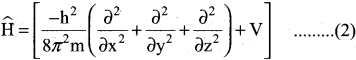

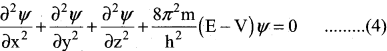
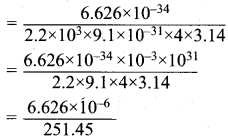
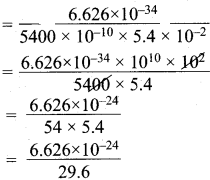

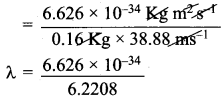
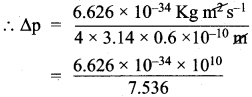
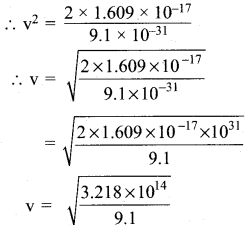
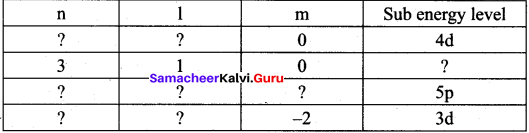
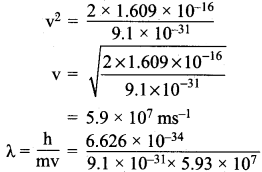
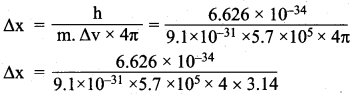




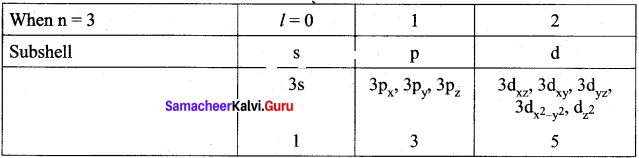
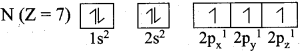
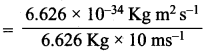
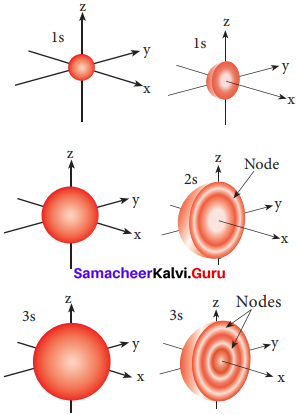
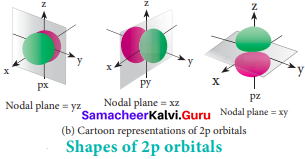
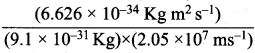 =355 x 10-4m.
=355 x 10-4m.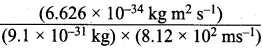
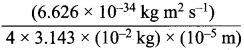 = 5.27 x 10-28mv
= 5.27 x 10-28mv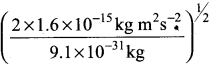 = 5.93 x 107m-1
= 5.93 x 107m-1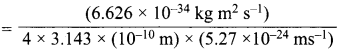 = 0.1 kg.
= 0.1 kg.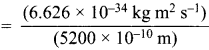
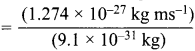 = 1.4 x 103 ms-1
= 1.4 x 103 ms-1