Tamilnadu Samacheer Kalvi 12th Chemistry Notes Chapter 5 Coordination Chemistry Notes
Double Salts:
- Addition compounds are formed when stoichiometric amounts of two or more stable compounds combine together.
- The Compounds which exist only in the solid state but dissociate into their constituent simple ions when dissolved in water. Such addition compounds are called double salts.
- For example, Mohr’s Salt – FeSO4.(NH4)2SO4.6H2O Potash alum – K2SO4.Al2(SO4)3.24 H2O
Coordination Compounds :
- The transition metals have a tendency to form complexes. The name is derived from the Latin words “complexus” and “coordinate” which mean “hold” and “to arrange” respectively.
- The compounds which retain their identity even in the solution, and have properties entirely different from those of the constituent simple ions are called coordination compounds.
- For example, [Cu(NH3)4]SO4 is a typical coordination compound.
Coordination Entity :
Coordination entity is an ion or a neutral molecule, composed of a central atom, usually a metal and the array of other atoms or groups of atoms (ligands) that are attached to it. In the formula the coordination entity is enclosed in sequare brackets. For example, in potassium ferrocyanide, K4[Fe(CN)6], the coordination entity is [Fe(CN)6]4-
Central metal atom / ion :
The central atom/ion is the one that occupies the central position in a coordination entity and binds other atoms or groups of atoms (ligands) to itself, through a coordinate covalent bond. For example, in K4[Fe(CN)6] the central metal ion is Fe2+.
Ligands :
The ligands are the atoms or groups of atoms bound to the central atom/ion. The atom in a ligand that is bound directly to the central metal atom is known as a donor atom. For example, in K4[Fe(CN)6] the ligand is CN– ion.
Coordination sphere :
The complex ion of the coordination compound containing the central metal atom/ion and the ligands attached to it, is collectively called coordination sphere and are usually enclosed in square brackets with the net charge. For example, the coordination compound K4[Fe(CN)6] contains the complex ion [Fe(CN)6]4- and is referred as the coordination sphere.
Coordination polyhedron :
The three dimensional spacial arrangement of ligand atoms/ ions that are directly attached to the central atom is known as the coordination polyhedron. For example, in K4[Fe(CN)6] the coordination polyhedra is octahedral. The coordination polyhedra of [Ni(CO)4] is tetrahedral.
Coordination number :
The number of ligand donor atoms bonded to a central metal ion in a complex is called the coordination number of the metal. For example, in K4[Fe(CN)6] the coordination number of Fe2+ is 6.
Oxidation state :
The oxidation state of a central atom in a coordination entity is defined as the charge it would bear if all the ligands were removed along with the electron pairs that were shared with the central atom. For example, in the coordination entity [Fe(CN)6]4- the oxidation state of iron is represented as (II).
Types of complexes :
The coordination compounds can be classified into the following types based on,
(i) the net charge of the complex ion
(ii) kinds of ligands present in the coordination entity.
- Cationic complex : Carries a net positive charge. E.g: [Ag(NF3)2]–
- Anionic complex : Carries a net negative charge. E.g: [Ag(CN)2)]–
- Neutral complex : Bears no net charge. E.g: [Ni(CO)4].
Isomerism :
It is the phenomenon in which more than one coordination compounds having the same molecular formula have different physical and chemical properties due to different arrangement of ligands around the central metal atom.
Structural isomers :
The coordination compounds with same formula, but have different connections among their constituent atoms are called structural isomers or constitutional isomers.
Linkage isomers :
This type of isomers arises when an ambidentate ligand is bonded to the central metal atom/ion through either of its two different donor atoms.
Coordination isomers :
This type of isomers arises in the coordination compounds having both cation and anion as complex ions. The interchange of one or more ligands between the cationic and the anionic coordination entities result in different isomers.
Ionisation isomers :
This type of isomers arises when an ionisable counter ion itself can act as a ligand. The exchange of such counter ions with one or more ligands in the coordination entity will results in ionisation isomers.
Solvate isomers :
The exchange of free solvent molecules such as water, ammonia, alcohol etc. in the crystal lattice with a ligand in the coordination entity will give different isomers. These type of isomers are called solvate isomers.
Stereoisomers :
The stereoisomers of a coordination compound have the same chemical formula and connectivity between the central metal atom and the ligands. But they differ in the spatial arrangement of ligands in three dimensional space.
Geometrical isomers :
Geometrical isomerism exists in heteroleptic complexes due to different possible three dimensional spatial arrangements of the ligands around the central metal atom.
Optical isomerism :
Coordination compounds which possess chairality exhibit optical isomerism similar to organic compounds. The pair of two optically active isomers which are mirror images of each other are called enantiomers. Their solutions rotate the plane of the plane polarised light either clockwise or anti clockwise and the corresponding isomers are called’d’ (dextrorotatory) and T (levorotatory) forms respectively.
Homoleptic complex :
The central metal ion / atom is coordinated to only one kind of ligands is called a homoleptic complex. E.g: [Co(NH3)6]3+, [Fe(H2O)6]2+.
Heteroleptic complex :
The central metal ion/atom is coordinated to more than one kind of ligands is called a heteroleptic complex. E.g:[Co(NH3)5Cl]2+, [Pt(NH3)Cl2].
Crystal field splitting power of various ligands :
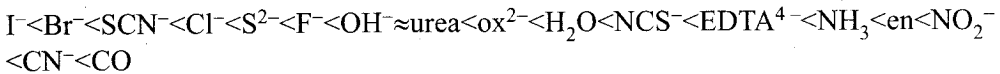
The above series is known as spectrochemical series.
Strong field ligands : The ligands present on the right side of the spectrochemical series such as carbonyl causes relatively larger crystal field splitting and are called strong field ligands.
Weak field ligands : The ligands present on the left side of the spectrochemical series such as iodide causes relatively smaller crystal fields splitting and are called weak fields ligands
Colour of the complex: Most of the transition metal complexes are coloured. A substance exhibits colour when it absorbs the light of a particular wavelength in the visible region and transmit the rest of the visible light. When this transmitted light enters our eye, our brain recognises its colour. The colour of the transmitted light is given by the complementary colour of the absorbed light.
Metallic carbonyls : Metal carbonyls are the transition metal complexes of carbon monoxide, containing metal-carbon bond. In these complexes CO molecule acts as a neutral ligand.
Synergic effect : In metal carbonyls, electron density moves from ligands to metal through sigma bonding and from metal to ligand through Pi-bonding. This is called synergic effect.
Labile complexes : In some cases, complexes can undergo rapid ligand substitution, such complexes are called labile complexes.
Inert complexes : In some cases, complexes undergo ligand substitution very slowly (or sometimes no substitution). Such complexes are called inert complexes.
Stability constant: The stability of a coordination complex is a measure of its resistance to the replacement of one ligand by another. The stability of a complex refer to the degree of association between two species involved in an equilibrium.
C is platin : It is a square planar coordination complex cis – [Pt(NH3)2Cl2], in which two similar ligands are in adjacent positions. It is a platinum based anticancer drug.
Coordination number : The number of ligand donor atoms bonded to central metal ion in a complex is called the coordination number of the metal.
Naming the central metal:
| Element | Name of the metal in | |
| Cation complex | Anionic complex | |
| Cr | Chromium | Chromate |
| Zn | Zinc | Zincate |
| A1 | Aluminium | Aluminate |
| Fe | Iron | Ferrate |
| Cu | Copper | Cuperate |
| Co | Cobalt | Cobaltate |
| Pb | Lead | Plumbate |
| Ag | Silver | Argentate |
| Sn | Tin | Stannate |
| Au | Gold | Aurate |
| Pt | Platinum | Platinate |
List of absorbed wavelength and their complementary colour
| Wave length(λ) of absorbed light (Å) | Wave number(v) of the absorbed light (cm-1) | Colour of absorbed light | Observed Colour |
| 4000 | 25000 | Violet | Yellow |
| 4750 | 21053 | Blue | Orange |
| 5100 | 19608 | Green | Red |
| 5700 | 17544 | Yellow | Violet |
| 5900 | 16949 | Orange | Blue |
| 6500 | 15385 | Red | Green |