Enhance your subject knowledge with Tamilnadu State Board for Chapter 8 Physical and Chemical Equilibrium and learn all the underlying concepts easily. Make sure to Download Samacheer Kalvi 11th Chemistry Book Solutions, Notes Pdf Chapter 8 Physical and Chemical Equilibrium Questions and Answers PDF on a day to day basis and score well in your exams. Are given after enormous research by people having high subject knowledge. You can rely on them and prepare any topic of Chemistry as per your convenience easily.
Tamilnadu Samacheer Kalvi 11th Chemistry Solutions Chapter 8 Physical and Chemical Equilibrium
Students looking for Chapter 8 Physical and Chemical Equilibrium Concepts can find them all in one place from our Tamilnadu State Board Solutions. Simply click on the links available to prepare the corresponding topics of Chemistry easily. Samacheer Kalvi 11th Chemistry Chapter wise Questions and Answers are given to you after sample research and as per the latest edition textbooks. Clarify all your queries and solve different questions to be familiar with the kind of questions appearing in the exam. Thus, you can increase your speed and accuracy in the final exam.
Samacheer Kalvi 11th Chemistry Chapter 8 Physical and Chemical Equilibrium Textual Evaluation Solved
Samacheer Kalvi 11th Chemistry Physical and Chemical Equilibrium Multiple Choice Questions
Question 1.
If Kb and Kf for a reversible reactions are 0.8 x 10-5 and 1.6 x 10-4 respectively, the value of the equilibrium constant is
(a) 20
(b) 0.2 x 10-1
(c) 0.05
(d) None of these
Answer:
(a) 20
Solution:

Question 2.
At a given temperature and pressure, the equilibrium constant values for the equilibria
![]()
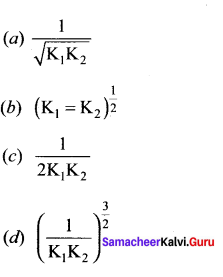
The relation between K1 and K2 is
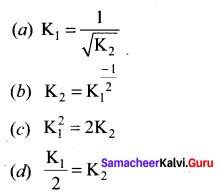
Answer:
![]()
Solution:
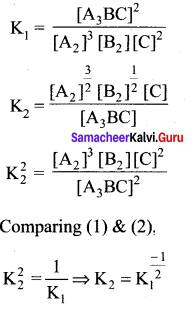
Question 3.
The equilibrium constant for a reaction at room temperature is K1 and that at 700 K is K2. If K1 > K2 then
(a) The forward reaction is exothermic
(b) The forward reaction is endothermic
(c) The reaction does not attain equilibrium
(d) The reverse reaction is exothermic
Answer:
(a) The forward reaction is exothermic
Solution:
T1 = 25 + 273 = 298 K, T2 = 700 K
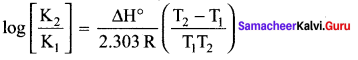
In this case, T2 > T1 and K1 > K2
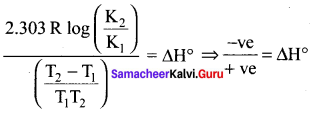
ΔH° is – ve i.e., forward reaction is exothermic.
Question 4.
The formation of ammonia from N2(g) and H2(g) is a reversible reaction
N2(g) + 3H2(g) \(\rightleftharpoons\) 2NH3(g) + Heat
What is the effect of increase of temperature on this equilibrium reaction
(a) equilibrium is unaltered
(b) formation of ammonia is favoured
(c) equilibrium is shifted to the left
(d) reaction rate does not change
Answer:
(c) equilibrium is shifted to the left
Solution:
Increase in temperature, favours the endothermic reaction, given that formation of NH3 is exothermic i.e., the reverse reaction is endothermic.
∴ Increase in temperature, shift the equilibrium to left
Question 5.
Solubility of carbon dioxide gas in cold water can be increased by ………….
(a) increase in pressure
(b) decrease in pressure
(c) increase in volume
(d) none of these
Answer:
(a) increase in pressure
Solution:
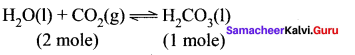
increase in pressure, favours the forward reaction.
Question 6.
Which one of the following is incorrect statement?
(a) for a system at equilibrium, Q is always less than the equilibrium constant
(b) equilibrium can be attained from either side of the reaction
(c) presence of catalyst affects both the forward reaction and reverse reaction to the same extent
(d) Equilibrium constant varied with temperature
Answer:
(a) for a system at equilibrium, Q is always less than the equilibrium constant
Solution:
Correct statement is, for a system at equilibrium, Q = Keq
Question 7.
K1 and K2 are the equilibrium constants for the reactions respectively.
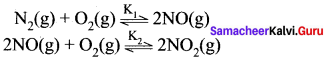
What is the equilibrium constant for the reaction NO2(g) \(\rightleftharpoons\) \(\frac { 1 }{ 2 }\)N2(g) + O2(g)
Answer:
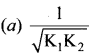
Solution:
Let equilibrium constant for the required reaction be K. Then,
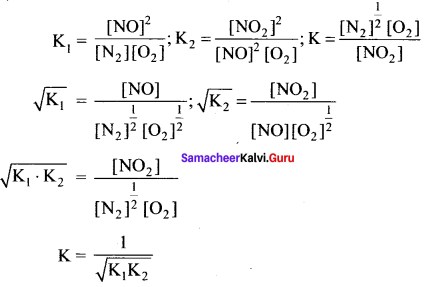
Question 8.
In the equilibrium, 2A(g) \(\rightleftharpoons\) 2B(g) + C2(g) the equilibrium concentrations of A, B and C, at 400 K are 1 x 104 M, 2.0 x 10-3 M, 1.5 x 10-4 M respectively. The value of Kc. for the equilibrium at 400 K is
(a) 0.06
(b) 0.09
(c) 0.62
(d) 3 x 10-2
Answer:
(a) 0.06
Solution:
[A] = 1 x 10-4 M
[B] = 2 x 10-3 M
[C] = 1.5 x 10-4 M
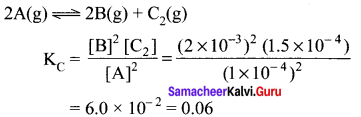
Question 9.
An equilibrium constant of 3.2 x 10-6 for a reaction means, the equilibrium is ………
(a) largely towards forward direction
(b) largely towards reverse direction
(c) never established
(d) none of these
Answer:
(b) largely towards reverse direction
Solution:

Question 10.
\(\frac { { K }_{ c } }{ { K }_{ p } }\) for the reaction, N2(g) + 3H2(g) \(\rightleftharpoons\) 2NH3(g) is ………
(a) \(\frac { 1 }{ RT }\)
(b) \(g\sqrt { RT } \)
(c) RT
(d) (RT)2
Answer:
(d) (RT)2
Solution:
N2(g) + 3H2(g) \(\rightleftharpoons\) 2NH3(g)
∆ng = 2 – 4 = – 2
Kp = Kc (RT)-2
\(\frac { { K }_{ c } }{ { K }_{ p } }\) = (RT)2
Question 11.
For the reaction, AB(g) \(\rightleftharpoons\) A(g) + B(g), at equilibrium, AB is 20% dissociated at a total pressure of R The equilibrium constant K is related to the total pressure by the expression ………..
(a) P = 24 Kp
(b) P = 8 Kp
(c) 24 p = Kp
(d) none of these
Answer:
(a) P =24 Kp
Solution:
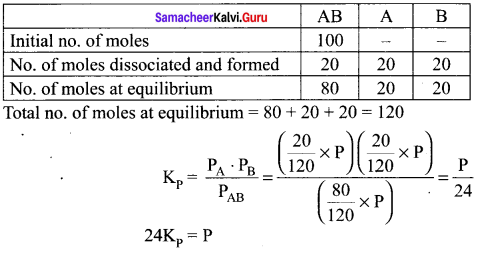
Question 12.
In which of the following equilibrium, K and K are not equal?
(a) 2NO(g) \(\rightleftharpoons\) N2(g) + O2(g)
(b) SO2(g) + NO2(g) \(\rightleftharpoons\) SO3(g) + NO(g)
(c) H2(g) + I2(g) \(\rightleftharpoons\) 2HI(g)
(d) PCI5(g) \(\rightleftharpoons\) PCl3(g) + Cl2(g)
Answer:
(d) PCI5(g) \(\rightleftharpoons\) PCl3(g) + Cl2(g)
Solution:
For reactions given in options (a), (b) and (c) ∆ng = 0
For option (d) ∆ng = 2 – 1 = 1
∴ Kp = Kc (RT)
Question 13.
If x is the fraction of PCI5 dissociated at equilibrium in the reaction,
PCl5 \(\rightleftharpoons\) PCl3 + Cl2
then starting with 0.5 moIe of PCI5 the total number of moles of reactants and products at equilibrium is
(a) 0.5 – x
(b) x + 0.5
(e) 2x + 0.5
(d) x ± 1
Answer:
(b) x + 0.5
Solution:

Total no. of moles at equilibrium = 0.5 – x + x + x = 0.5 + x
Question 14.
The values of Kp1 and Kp2 for the reactions X \(\rightleftharpoons\) Y + Z and A \(\rightleftharpoons\) 2B are in the ratio 9 : 1 if degree of dissociation and initial concentration of X and A be equal then total pressure at equilibrium P1, and P2 are in the ratio
(a) 36 : 1
(b) 1: 1
(c) 3 : 1
(d) 1: 9
Answer:
(a) 36: 1
Solution:
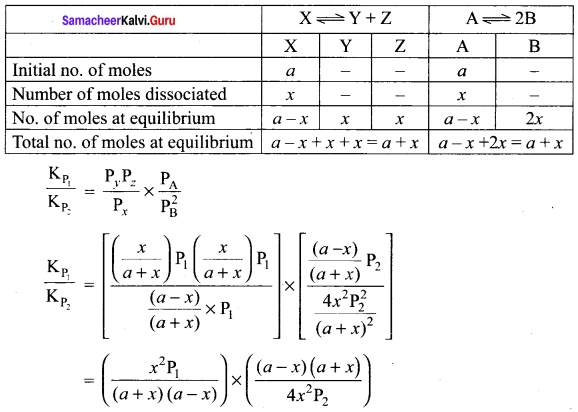
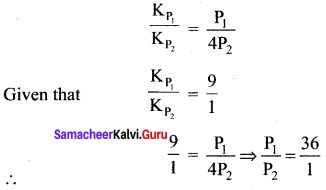
Question 15.
In the reaction, Fe(OH)3(s) \(\rightleftharpoons\) Fe3+(aq) + 3OH–(aq), if the concentration of OH– ions is decreased by ¼ times, then the equilibrium concentration of Fe3+ will …………
(a) not changed
(b) also decreased by ¼ times
(c) increase by 4 times
(d) increase by 64 times
Answer:
(d) increase by 64 times
Solution:
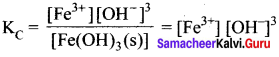
[∵ Concentration of solids is constant)
When concentration of OH– ions decreased by \(\frac { 1 }{ 4 }\) times, then
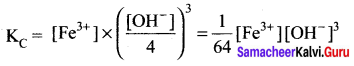
To maintain KC as constant, concentration of Fe3+ will increase by 64 times.
Question 16.
Consider the reaction where Kp = 0.5 at a particular temperature
PCI5(g) \(\rightleftharpoons\) PCI3(g) + Cl2(g) if the three gases are mixed in a container so that the partial pressure of each gas is initially 1 atm, then which one of the following is true?
(a) more PCI3 will be produced
(b) more Cl2 will be produced
(c) more PCI5 will be produced
(d) none of these
Answer:
(c) more PCI5 will be produced .
Solution:
Kp = 0.5
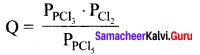
Q = \(\frac { 1 x 1 }{ 1 }\)
Q > Kp
∴Reverse reaction is favoured; i.e., more PCI5 will be produced.
Question 17.
Equimolar concentrations of H2 and I2 are heated to equilibrium in a 1 litre flask. What percentage of initial concentration of H2 has reacted at equilibrium if rate constant for both forward and reverse reactions are equal
(a) 33%
(b) 66%
(c)(33)2 0/0
(d) 16.5%
Answer:
(a) 33%
Solution:
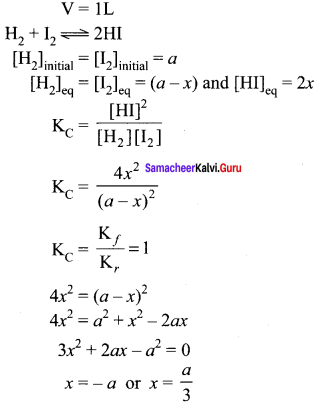
As degree of dissociation cannot be negative. Therefore, degree of dissociation
= \(\frac { a }{ 3 }\) x 100 = 33.33%
Question 18.
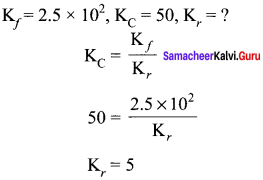
In a chemical equilibrium, the rate constant for the forward reaction is 2.5 x 1 and the equilibrium constant is 50. The rate constant for the reverse reaction is ……….
(a) 11.5
(b) 5
(c) 2 x 102
(d) 2 x 103
Answer:
(b) 5
Solution:
Question 19.
Which of the following is not a general characteristic of equilibrium involving physical process
(a) Equilibrium is possible only in a closed system at a given temperature
(b) The opposing processes occur at the same rate and there is a dynamic but stable condition
(c) All the physical processes stop at equilibrium
(d) All measurable properties of the system remains constant
Answer:
(c) All the physical processes stop at equilibrium
Solution:
Correct statement – Physical processes occurs at the same rate at equilibrium.
Question 20.
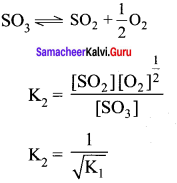
For the fórmation of two moles of SO3(g) from SO2 and O2, the equilibrium constant is K1. The equilibrium constant for the dissociation of one mole of SO3 into SO2 and O2 is …………
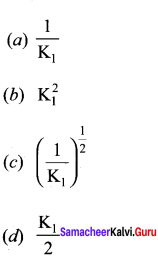
Answer:
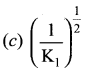
Solution:
2SO2 +O2 \(\rightleftharpoons\) 2SO3
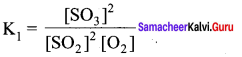
Dissociation of 1 mole of 2SO3
Question 21.
Match the equilibria with the corresponding conditions …………
(i) Liquid \(\rightleftharpoons\) Vapour – 1. Melting point
(ii) Solid \(\rightleftharpoons\) Liquid – 2. Saturated solution
(iii) Solid \(\rightleftharpoons\) Vapour – 3. Boiling point
(iv) Solute (s) \(\rightleftharpoons\) Solute (Solution) – 4. Sublimation point
5. Unsaturated solution

Answer:
(b) 3, 1, 4, 2
Question 22.
Consider the following reversible reaction at equilibrium, A + B \(\rightleftharpoons\) C, if the concentration of the reactants A and B are doubled, then the equilibrium constant will ………
(a) be doubled
(b) become one fourth
(c) be halved
(d) remain the same
Answer:
(d) remain the same
Solution:
A + B \(\rightleftharpoons\) C
KC = \(\frac { [C] }{ [A] [B] }\)
If [A] and [B] are doubled, [C] increases 4 times to maintain KC as constant.
∴ Equilibrium constant will remain the same.
Question 23.
[CO(H2O)6]2+ (aq) (pink) + 4C– (aq) \(\rightleftharpoons\) [COCI4]2- (aq) (blue) + 6H2O (1) In the above reaction at equilibrium, the reaction mixture is blue in colour at room temperature. On cooling this mixture, it becomes pink in colour. On the basis of this information, which one
of the following is true?
(a) ∆H > 0 for the forward reaction
(b) ∆H = 0 for the reverse reaction
(c) ∆H < 0 for the forward reaction
(d) Sign of the ∆H cannot be predicted based on this information.
Answer: (a) ∆H > 0 for the forward reaction
Solution:
On cooling, reverse reaction predominates and the solution is pink in colour. Decrease in temperature, favours the reverse reaction i.e. reverse reaction is exothermic (∆H < 0)and for the forward reaction is endothermic (∆H > 0).
Question 24.
The equilibrium constants of the following reactions are:
N2 + 3H2 \(\rightleftharpoons\) 2NH3 ; K1
N2 + O2 \(\rightleftharpoons\) 2NO ; K2
H2+ \(\frac { 1 }{ 2 }\)O2 \(\rightleftharpoons\) H2O ; K3
The equilibrium constant (K) for the reaction; ![]() will be
will be

Answer:
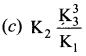
Solution:
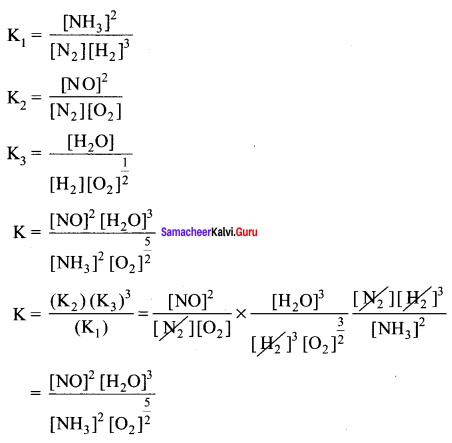
Question 25.
A 20 litre container at 400 K contains CO2(g) at pressure 0.4 atm and an excess of SrO (neglect the volume of solid SrO). The volume of the container is now decreased by moving the movable piston fitted in the container. The maximum volume of the container, when pressure of CO2 attains its maximum value will be.
Given that: SrCO3(s) \(\rightleftharpoons\) SrO(s) + CO2(g)
(a) 2 litre
(b) 5 litre
(c) 10 Litre
(d) 4 litre
Answer:
(b) 5 litre
Solution:
Given that Kp = 1.6 atm
V1 = 20 L
V2 = ?
T1 = 400 K
T2 = 400 K
Kp = Pco2
Pco2 = 1.6 atm
P1 = 0.4 atm
P2 = 1.6 atm

Samacheer Kalvi 11th Chemistry Physical and Chemical Equilibrium Short Answer Questions
Question 26.
If there is no change in concentration, why is the equilibrium state considered dynamic?
Answer:
At chemical equilibrium the rate of two opposing reactions are equal and the concentration of. reactants and products do not change with time. This condition is not static and is dynamic, because both the forward and reverse reactions are still occurring with the same rate and no macroscopic change is observed. So chemical equilibrium is in a state of dynamic equilibrium.
Question 27.
For a given reaction at a particular temperature, the equilibrium constant has constant value. Is the value of Q also constant? Explain.
Answer:
In the chemical reaction, as the reaction proceeds, there is a continuous change in the concentration of reactants and products and also the Q value until the reaction reaches the equilibrium. So even at particular temperature, Q is not constant. Even once the equilibrium is achieved then change in concentration of reactants or products, pressure, volume will change the value of Q.
Question 28.
What is the relation between Kp and KC. Give one example for which Kp is equal to KC.
Answer:
The relation between Kp and Kc is Kp = KC (RT)∆ng
Kp = equilibrium constant is terms of partial pressure.
Kc = equilibrium constant is terms of concentration.
R = gas constant
T = Temperature.
∆ng = Difference between the sum of the number of moles of products and the sum of number of moles of reactants in the gas phase. When ∆ng = 0
Kp = KC(RT)0 = KC i.e., Kp = KC
Example: H2(g) + I2(g) \(\rightleftharpoons\) 2HI(g)
∆ng = 2 – 2 = 0
∴ Kp = KC for the synthesis of HI.
Question 29.
For a gaseous homogeneous reaction at equilibrium, number of moles of products are greater than the number of moles of reactants. Is KC is larger or smaller than Kp ?
Answer:
For a homogeneous reaction at equilibrium, number of moles of products (np) are greater than the number of moles of reactants (nR) then ∆ng = + ve
np > nR
∆ng = + ve
If ∆ng is ve, Kp value is greater than KC
Kp = KC. (RT)+ve
Kp > KC
Example: PCl5 \(\rightleftharpoons\) PCl3(g) + Cl2(g)
2 – 1 = 1
Kp = KC (RT)1
Kp > KC
Question 30.
When the numerical value of the reaction quotient (Q) is greater than the equilibrium constant (K), in which direction does the reaction proceed to reach equilibrium?
Answer:
When Q > KC the reaction will proceed in the reverse direction, i.e, formation of reactants.
Question 31.
For the reaction: A2(g) + B2(g) \(\rightleftharpoons\) 2AB(g); ∆H is – ve.
Answer:
The following molecular scenes represent different reaction mixture
(A – dark grey, B – light grey)
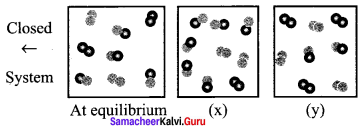
- Calculate the equilibrium constant Kp and KC
- For the reaction mixture represented by scene (x), (y) the reaction proceed in which directions?
- What is the effect of increase in pressure for the mixture at equilibrium?
Answer:
1. 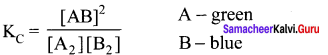
At equilibrium
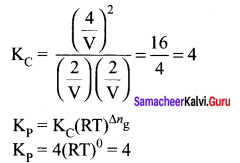
2.
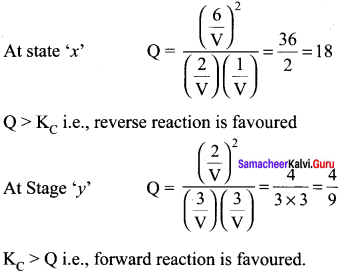
3. Since ∆ng = 2 – 2 = 0, thus, pressure has no effect. So by increasing the pressure, equilibrium will not be affected.
Question 32.
State Le – Chatelier principle.
Answer:
It states that If a system at equilibrium is disturbed, then the system shifts itself in a direction that nullifies the effect of that disturbance.
Question 33.
Consider the following reactions
- H2(g) + l2(g) \(\rightleftharpoons\) 2HI(g)
- CaCO3(s) \(\rightleftharpoons\) CaO(s) + CO2(g)
- S(s) + 3F2(g) \(\rightleftharpoons\) SF6(g)
In each of the above reaction find out whether you have to increase (or) decrease the volume to increase the yield of the product.
Answer:
1. H2(g) + l2(g) \(\rightleftharpoons\) 2HI(g)
In the above equilibrium reaction, volume of gaseous molecules is equal on both sides. So increase or decrease the volume will not affect the equilibrium and there will be no change in the yield of product.
2. ![]()
Volume is greater in product side. By decreasing the pressure, volume will increase thus, to get more of product CO2, the pressure should be decreased or volume should be increased.
3. ![]()
Volume is lesser in product side. So by increasing the pressure, equilibrium shifts to the product side.
Question 34.
State law of mass action.
Answer:
The law states that “At any instant, the rate of chemical reaction at a given temperature is directly proportional to the product of the active masses of the reactants at that instant.”
Question 35.
Explain how will you predict the direction of an equilibrium reaction.
Answer:
- A large value of KC indicates that the reaction reaches equilibrium with high product yield.
- A low value of KC indicate that the reaction reaches equilibrium with low product formed.
- In general, if the K is greater than I the reaction proceeds nearly to completion. If is less than 10-3, the reaction rarely proceeds.
- If K < 10-3, reverse reaction is favoured. If Kc > 103, forward reaction is favoured.
Samacheer Kalvi 11th Chemistry Physical and Chemical Equilibrium Long Answer Questions
Question 36.
Derive a general expression for the equilibrium constant Kp and KC for the reaction.
3H2(g) + N2(g) \(\rightleftharpoons\) 2NH3(g)
Answer:
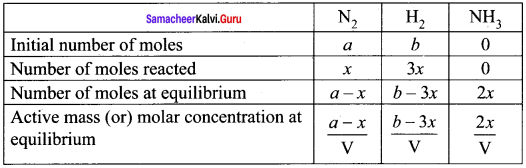
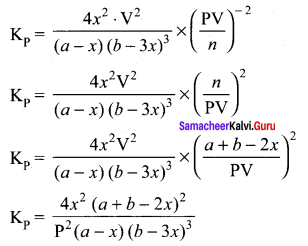
In the formation of ammonia, ‘a’ moles of Nitrogen and ‘b’ moles of hydrogen gas are allowed to react in a container of volume of ‘V’. Let ‘x’ moles of nitrogen react with 3x moles of hydrogen to give 2x moles of ammonia.
N2(g) + 3H2(g) \(\rightleftharpoons\) 2NH3(g)
Applying law of mass action
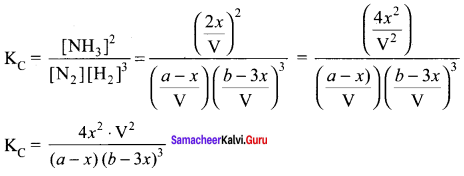
Total number of moles at equilibrium n = a – x + b -3x + 2x = a + b – 2x

Total number of moles at equilibrium
n = a – x + b – 3x + 2x = a + b – 2x
Question 37.
Write a balanced chemical equation for an equilibrium reaction for which the equilibrium constant is given by expression?
Answer:
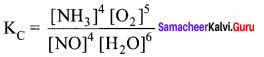
Question 38.
What is the effect of added inert gas on the reaction at equilibrium at constant volume?
Answer:
When an inert gas (i.e., a gas which does not react with any other species involved in equilibrium) is added to an equilibrium system at constant volume, the total number of moles. of gases present in the container increases, that is, the total pressure of gases increases, the partial pressure of the reactants and the products are unchanged. Hence at constant volume, addition of inert gas has no effect on equilibrium.
Question 39.
Derive the relation between Kp and KC.
Answer:
Consider a general reaction in which all reactants and products are ideal gases.
x A + y B \(\rightleftharpoons\) lC+mD
The equilibrium constant KC is
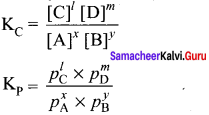
The ideal gas equation is
PV = nRT or P = \(\frac { n }{ V }\) RT
Since,
Active mass = molar concentration = \(\frac { n }{ V }\)
P = Active mass x RT
Based on the above expression, the partial pressure of the reacants and products can be expressed as

On substitution in equation (2),

By comparing equation (1) and (4), we get
Kp = KC (RT) ∆ng
where ∆ng is the difference between the sum of number of moles of products and the sum of number of moles of reactants in the gas phase.
(i) If ∆ng = 0, Kb = KC(RT)0
Kp = KC
Example: H2(g) + I2 \(\rightleftharpoons\) 2HI(g)
(ii) where
∆ng = +Ve
Kp = KC (RT)+ve
Kp = KC
Example: 2NH3(g) N2(g) + 3H2(g)
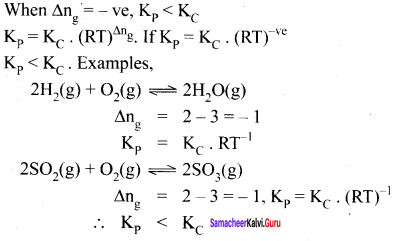
(iii) When
∆ng = -Ve
Kp = KC (RT)-ve
Kp < KC
Example: 2SO2(g) +O2(g) \(\rightleftharpoons\) 2SO3(g)
Question 40.
One mole of PCl5 is heated in one litre closed container. If 0.6 mole of chlorine is found at equilibrium, calculate the value of equilibrium constant.
Answer:
Given that
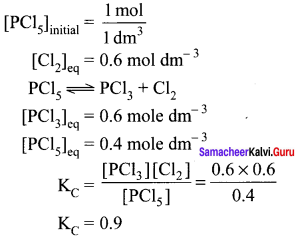
Question 41.
For the reaction: SrCO2(s) \(\rightleftharpoons\) SrO (s) + CO2(g), the value of equilibrium constant Kp = 2.2 x 10-4 at 1002K. Calculate KC for the reaction.
Answer:
For the reaction, SrCO2(s) \(\rightleftharpoons\) SrO (s) + CO2(g)
∆ng = 1 – 0 = 1
Kp = Kp (RT)
2.2 x 10-4 = KC (0.0821)(1002)
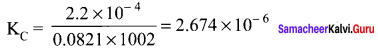
Question 42.
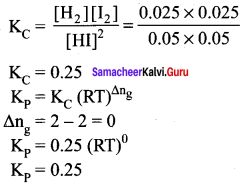
To study the decomposition of hydrogen iodide, a student fills an evacuated 3 litre flask with 0.3 mol of HI gas and allows the reaction to proceed at 500°C. At equilibrium he found the concentration of HI which is equal to 0.05 M. Calculate Kp and KC for this reaction.
Answer:
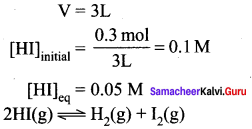

Question 43.
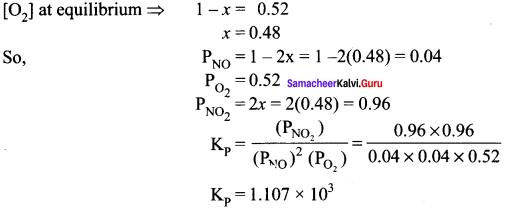
Oxidation of nitrogen monoxide was studied at 200°C with initial pressures of 1 atm NO and 1 atm of O2. At equilibrium partial pressure of oxygen is found to be 0.52 atm. Calculate Kp value.
Answer:
2NO(g) + O2(g) \(\rightleftharpoons\) 2NO2(g)

Question 44.
1 mol of CH4, 1 mole of CS2 and 2 mol of H2S are 2 moI of H2 are mixed in a 500 mL flask. The equilibrium constant for the reaction KC = 4 x 10-2 mol2 lit-2. In which direction will the reaction proceed to reach equilibrium?
Answer:
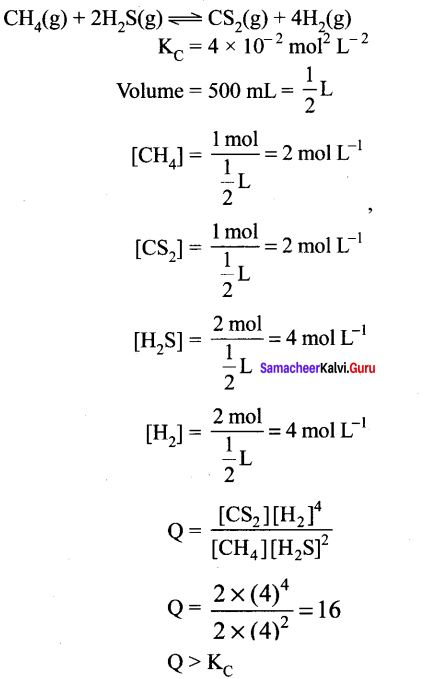
∴ The reaction will proceed in the reverse direction to reach the equilibrium.
Question 45.
At particular temperature KC = 4 x 10-2 for the reaction
H2S(g) \(\rightleftharpoons\) 2H2(g) + \(\frac { 1 }{ 2 }\) S2(g). Calculate KC for each of the following reaction
(i). H2S(g) \(\rightleftharpoons\) 2H2(g) + S2(g)
(ii). 3H2S(g) \(\rightleftharpoons\) 3H2(g) + \(\frac { 3 }{ 2 }\) S2(g)
Answer:
KC = 4 x 10-2 for the reaction
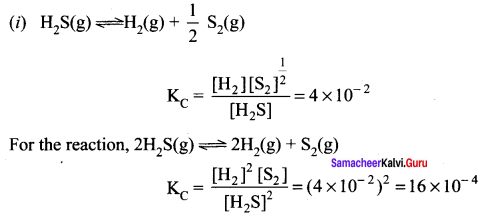
For the reaction 3H2S(g) \(\rightleftharpoons\) 3H2(g) + \(\frac { 3 }{ 2 }\) S2(g)
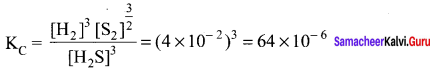
Question 46.
28 g of nitrogen and 6 g of hydrogen were mixed in a 1 litre closed container. At equilibrium 17g NH3 was produced. Calculate the weight of nitrogen, hydrogen at equilibrium.
Answer:
Given
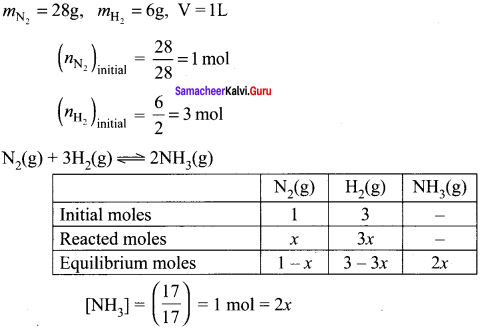
[NH3] = \((\frac { 17 }{ 17 })\) = 1 mol = 2x
x = 0.5 mol
At equilibrium, [N2] = 1 – x = 0.5 mol
[H2] = 3 – 3x = 3 – 3 (0.5) = 1.5 mol
Weight of N2 (no. of moles of N2) x molar mass of N2 = 0.5 x 28 = 14g
Weight of H2 = (no. of moles of H2) x molar mass of H2= 1.5 x 2 = 3g
Question 47.
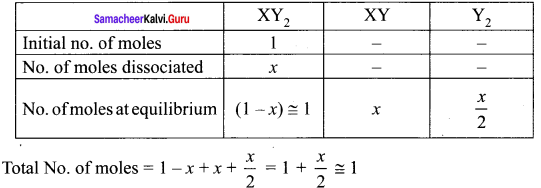
The equilibrium for the dissociation of XY2 is given as,
2XY2 (g) \(\rightleftharpoons\) 2XY (g) + Y2(g).
If the degree of dissociation x is so small compared to one. Show that 2 Kp = Px3 where P is the total pressure and K is the dissociation equilibrium constant of XY2.
Answer:
2XY2 (g) \(\rightleftharpoons\) 2XY (g) + Y2(g)
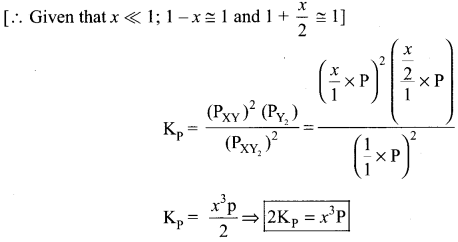
Question 48.
A sealed container was filled with I mol of A, (g), I mol B, (g) at 800 K and total pressure 1.00 bar. Calculate the amounts of the components in the mixture at equilibrium given that K = 1 for the reaction:
A2(g) + B2(g) \(\rightleftharpoons\) 2AB(g)
Answer:
A2(g) + B2(g) \(\rightleftharpoons\) 2AB(g)
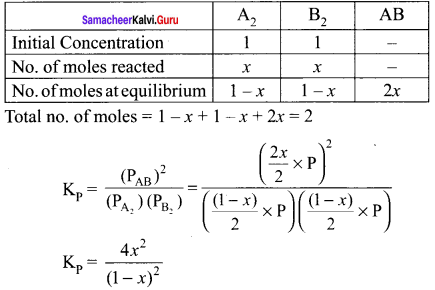
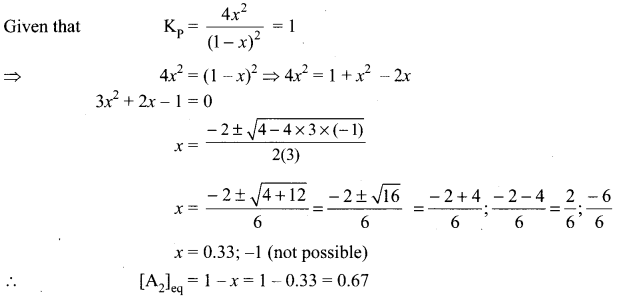
[B2]eq = 1 – x = 1 – 0.33 = 0.667
[AB]2 = 2x = 2 x 0.33 = 0.66
Question 49.
Deduce the Vant Hoff equation.
Answer:
This equation gives the quantitative temperature dependance of equilibrium constant K. The relation between standard free energy change ∆G° and equilibrium constant is
∆G° = – RT ln K …………(1)
We know that,
∆G° = ∆H° – T∆S° ………….(2)
Substituting (1) in equation (2)
– RT lnK = ∆H° – T∆S°
Rearranging, ln K = \(\frac { – ∆H° }{ RT }\) + \(\frac { ∆S° }{ R }\) ………….(4)
Differentiating equation (3) with respect to temperature
d(lnK) ∆H° \(\frac { d(lnK) }{ dT }\) = \(\frac { { \triangle H }^{ 0 } }{ { RT }^{ 2 } }\)
Equation (4) is known as differential form of van’t Hoff equation. On integrating the equation 4, between T1 and T2 with their respective equilibrium constants and K2
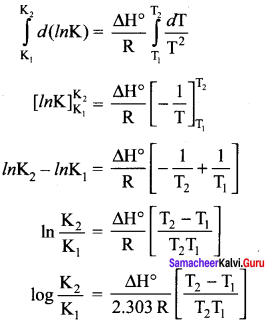
Equation is known as integrated form of van’t Hoff equation.
Question 50.
The equilibrium constant K for the reaction
N2(g) + 3H2(g) = 2NH3(g) is 8.19 x 102
at 298 K and 4.6 x 10-1 at 498 K. Calculate ∆H° for the reaction.
Answer:
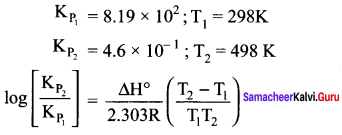

Question 51.
The partial pressure of carbon dioxide in the reaction CaCO3(s) \(\rightleftharpoons\) CaO (s) + CO2(g) is 1.017 x 10-3 atm at 500° C. Calculate Kp, at 600° C for the reaction. ∆H for the reaction is 181 kJ mol-1 and does not change in the given range of temperature.
Answer:
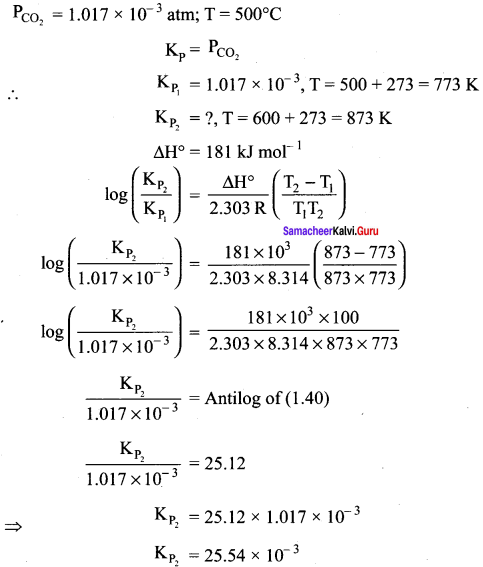
Samacheer Kalvi 11th Chemistry Physical and Chemical Equilibrium In – Text problems Solved
Question 1.
Consider the following reaction
Fe3+(aq) + SCN–(aq) [Fe(SCN)]2+(aq)
A solution is made with initial Fe3+, SCN– concentration of 1 x 10-3 M and 8 x 10-4M respectively. At equilibrium [Fe(SCN)]2+ concentration is 2 x 10-4M. Calculate the value of equilibrium constant.
Answer:
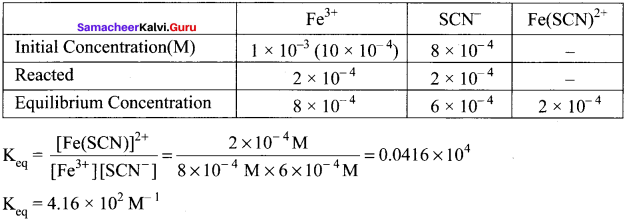
Question 2.
The atmospheric oxidation of NO
2NO(g) + O2(g) \(\rightleftharpoons\) 2NO2(g)
was studied with initial pressure of 1 atm of NO and I atm of O2. At equilibrium, partial pressure of oxygen is 0.52 atm. Calculate Kp of the reaction.
Answer:
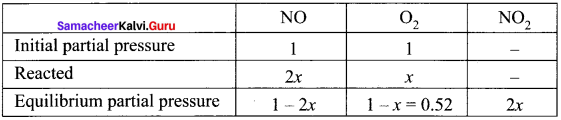
As,
1 – x = 0.52
x = 0. 48
= At equilibrium,
PNO = 1 – 2x = 1 – 2(0.48) = 0.04
PNO2 = 2x = 2(0.48) = 0.96
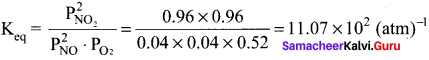
Question 3.
The following water gas shift reaction is an important industrial process for the production of hydrogen gas.
CO(g) + H2O(g) \(\rightleftharpoons\) CO2(g) + H2(g) At a given temperature Kp = 2.7. If 0.13 moI of CO, 0.56 moI of water, 0.78 mol of CO2 and 0.28 mol of H2 are introduced into a 2L flask, find out in which direction must the reaction proceed to reach equilibrium.
Answer:
CO(g) + H2O(g) \(\rightleftharpoons\) CO2(g) + H2(g)
Given
Kp = 2.7
[CO] = 0.13 mol, [H2O] = 0.56 mol
[CO2] = 0.78 mol; [H2] = 0.28 mol
V = 2L
Kp = KC (RT)Δng
2.7 = KC(RT)°
KC = 2.7
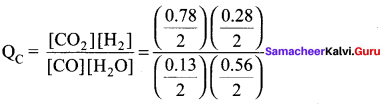
Q = 3
Q > KC Hence, the reaction proceed in the reverse direction.
Question 4.
1 moI of PCl5, kept in a closed container of volume 1 dm3 and was allowed to attain equilibrium at 423 K. Calculate the equilibrium composition of reaction mixture. (The KC value for PCl5 dissociation at 423 K is 2)
Answer:
PCl5 \(\rightleftharpoons\) PCL3 + Cl2
Given that [PCl5]initial = 1 mol
V = 1 dm3
KC = 2
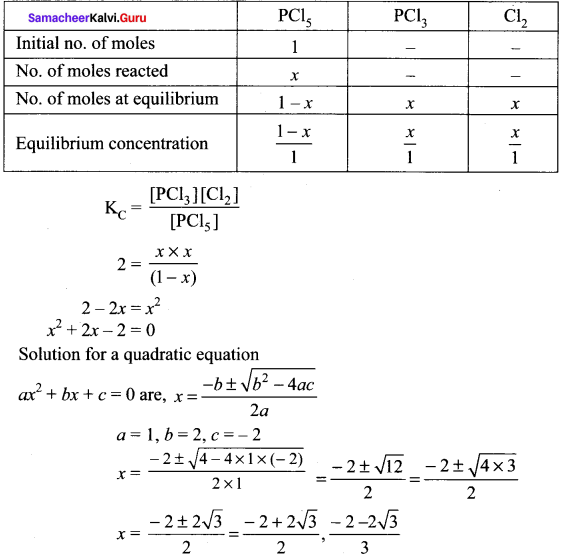
∴ Equilibrium concentration of
[PCl5]eq = \(\frac { 1-x }{ 1 }\) = 1 – 0.732 = 0.268 M
[PCl3]eq = \(\frac { x }{ 1 }\) = \(\frac { 0.732 }{ 1 }\) = 0.732
[Cl2]eq = \(\frac { x }{ 1 }\) = \(\frac { 0.732 }{ 1 }\) = 0.732
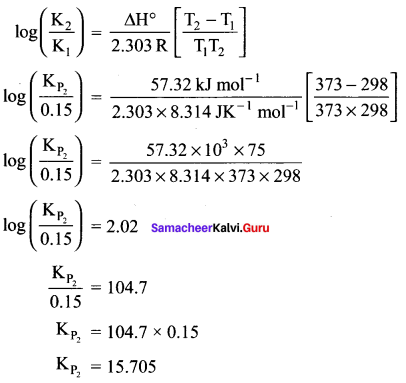
Question 5.
The equilibrium constant for the following reaction is 0.15 at 298 K and 1 atm pressure.
Answer:
N2O4(g) \(\rightleftharpoons\) 2NO2(g)
T1 = 298 K
Kp1 = 0.15
T2 = 1000C = 100 + 273 = 373 K
Kp2 = ?
Samacheer Kalvi 11th Chemistry Physical and Chemical Equilibrium In – Text problems Solved
Question 1.
One mole of H2 and one mole of I2 are allowed to attain equilibrium in 1 lit container. If the equilibrium mixture contains 0.4 mole of HI. Calculate the equilibrium constant.
Answer:
Given data: [H2] = I mole, [I2] = 1 mole
At equilibrium, [HI] = 0.4 mole, KC = ?
H2(g) + I2(g) \(\rightleftharpoons\) 2HI(g)
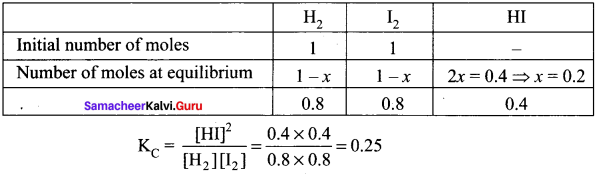
Question 2.
The equilibrium concentrations of NH3, N2 and H2 are 1.8 x 102 M, 1.2 x 10-2 M and 3 x 10-2 M respectively. Calculate the equilibrium constant for the formation of NH3 from N2 and H2. [Hint: M = mol lit-1]
Answer:
Given data:
[NH3] 1.8 x 10-2 M, [N2] = 1.2 x 10-2M, [H2] 3 x 10-2 M, KC = ?

Question 3.
The equilibrium constant at 298 K for a reaction is 100.
A+ B \(\rightleftharpoons\) C + D
If the initial concentration of all the four species is 1 M, the equilibrium concentration of D (in mol lit-1) will be
Answer:
Given data: [A] = [B] = [C] = [D] = 1 M, KC = 100, [D]eq = ?
Let x be the number of moles reactants reacted
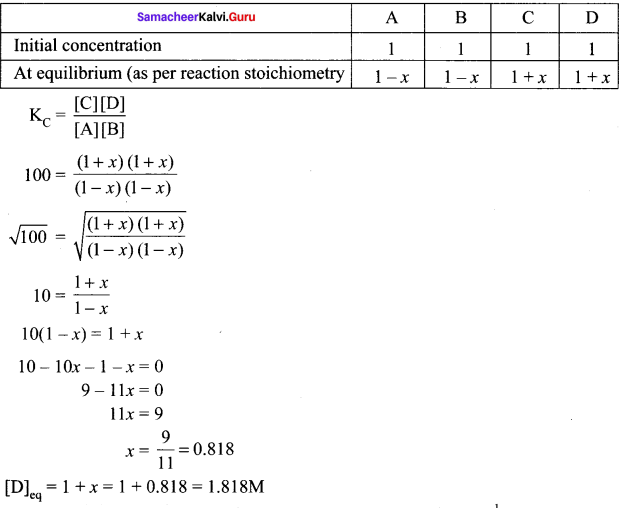
Question 4.
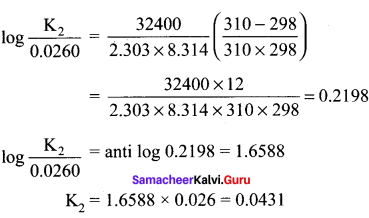
For an equilibrium reaction Kp = 0.0260 at 25° C and ?H = 32.4 kJ mor-1. Calculate Kp at 37° C.
Answer:
T1 = 25 + 273 = 298 K
T2 = 37 + 273 = 310 K
ΔH = 32.4 kJ mor-1 = 32400 J mol-1
R = 8.314 JK-1 mol-1
Kp1 = 0.0260
Kp2 = ?
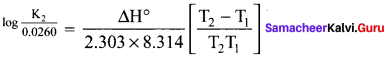
Samacheer Kalvi 11th Chemistry Physical and Chemical Equilibrium Additional Questions
I. Choose the correct answer.
Question 1.
Which of the following represents physical equilibrium?
(a) PCl5(g) \(\rightleftharpoons\) PCl3(g) + Cl2(g)
(b) H2(g) + I2(g) \(\rightleftharpoons\) 2HI(g)
(c) H2O(l) \(\rightleftharpoons\) H2O(g)
(d) N2(g) + 3H2(g) \(\rightleftharpoons\) 2NH3(g)
Answer:
(c) H2O(l) \(\rightleftharpoons\) H2O(g)
Solution:
Physical states arc in equilibrium i.e., liquid – vapour equilibrium.
Question 2.
Which one of the following is an example of chemical equilibrium?
(a) 2NO(g) + O2(g) \(\rightleftharpoons\) 2NO2(g)
(b) I2(s) \(\rightleftharpoons\) I2(g)
(c) H2O(s) \(\rightleftharpoons\) H2O(1)
(d) NH2CI(s) \(\rightleftharpoons\) NH4CI(g)
Answer:
(a) 2NO(g) + O2(g) \(\rightleftharpoons\) 2NO2(g)
Solution:
All the other three are physical equilibrium. Only (a) is chemical equilibrium.
Question 3.
Which one of the following does not undergo sublimation?
(a) Iodine
(b) water
(c) Camphor
(d) Ammonium chloride
Answer:
(b) Water
Question 4.
At chemical equilibrium,
(a) rate of forward reaction = rate of backward reaction
(b) rate of forward reaction > rate of backward reaction
(c) rate of forward reaction < rate of backward reaction
(d) rate of forward reaction = rate of backward reaction
Answer:
(a) rate of forward reaction = rate of backward reaction
Question 5.
Which of the following is an example of homogeneous equilibrium?
(a) H2O(1) \(\rightleftharpoons\) H2O(g)
(b) CaCO3(s) \(\rightleftharpoons\) CaO(s) + CO(g)
(c) H2(g) + I2(g) \(\rightleftharpoons\) 2HI(g)
(d) 2CO(g) \(\rightleftharpoons\) CO2(g) + C(s)
Answer:
(c) H2(g) + I2(g) \(\rightleftharpoons\) 2HI(g)
Solution:
Here all reactants and products are in same phase i.e., gaseous phase.
Question 6.
Which of the following is an example of heterogeneous equilibrium?
(a) Synthesis of HI
(b) Dissociation of PCI5
(c) Acid hydrolysis of ester
(d) Decomposition of limestone
Answer:
(d) Decomposition of limestone
Solution:
CaCO3(s) → CaO(s) + CO2(g)
Here CO2 is in gaseous state while CaCO3 and CaO are in solid state.
Question 7.
Statement I: In dissociation of PCI5 to PCI3 and CI2, Kp > KC
Statement II: In dissociation of PCI5, Δng = – ve and so Kp > KC.
(a) Statement I & II are correct and statement II is the correct explanation of statement I.
(b) Statement I & II are correct but statement II is not the correct explanation of statement I.
(c) Statement I is correct but statement II is wrong.
(d) Statement I is wrong but statement II is correct.
Answer:
(c) Statement I is correct but statement II is wrong.
Solution:
Δng = 2 – 1 = 1 = +ve
Question 8.
In the reaction, 2NH3(g) \(\rightleftharpoons\) N2(g) + 3H2(g)
(a) Kp = KC
(b) Kp < KC
(c) Kp > KC
(d) Kp = \(\frac { 1 }{ { K }_{ C } }\)
Answer:
(c) Kp > KC
Solution:
Kp = KC (RT)Δng and Δng = 4 – 2 = 2
∴ Kp = KC (RT)2 = Kp > KC
Question 9.
In which of the following reaction, Kp is equal to KC ?
(a) N2(g) ± O2(g) \(\rightleftharpoons\) 2NO(g)
(b) 2NH3(g) \(\rightleftharpoons\) N2(g) + 3H2(g)
(c) 2H2(g) + O2(g) \(\rightleftharpoons\) 2H2O(g)
(d) PCI5(g) \(\rightleftharpoons\) PCI3(g) + CI2
Answer:
(a) N2(g) ± O2(g) \(\rightleftharpoons\) 2NO(g)
Solution:
KC (RT)Δng when Δng = 0 then K = K for option (a), Δng = 2 – 2 = 0
Question 10.
In the equilibrium reaction CaCO3(s) \(\rightleftharpoons\) CaO(s) + CO2(g) whose concentration remains constant at a given temperature?
(a) CaO
(b) CO2
(e) CaCO3
(d) Both (a) and (c)
Answer:
(d) Both (a) and (c)
Solution:
Concentration of solids remains constant at a particular temperature.
Question 11.
Consider the following equilibrium reaction and relate their equilibrium constants
(i) N2 + O2 \(\rightleftharpoons\) 2NO; K1
(ii) 2NO + O2 \(\rightleftharpoons\) 2NO2; K2
(iii) N2 + 2O2 \(\rightleftharpoons\) 2NO2; K3
(a) K3 = K2 = K1
(b) K1 x K3 = K2
(c) K1 x K2 = K3
(d) \(\frac { { K }_{ 1 } }{ { K }_{ 2 } }\) = K3
Answer:
(c) K1 x K2 = K3
Solution:
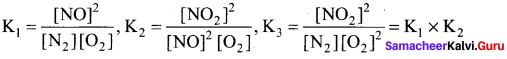
Question 12.
Statement I: A pure solid in an equilibrium reaction has the same concentration at a given temperature.
Statement II: The solid does not expand to fill its container and it has same number of moles of its volume.
(a) Statement I and II are correct and statement II is the correct explanation of statement of I.
(b) Statement I and II are correct but II is not the correct explanation of!.
(c) Statement I and II are not correct.
(d) Statement I is wrong but 11 is correct.
Answer:
(a) Statement I and II are correct and statement II is the correct explanation of statement of I.
Question 13.
Find the Q value of the reaction H2(g) + I2(g) \(\rightleftharpoons\) 2HI(g) at an instant where concentration of H2, I2 and HI are found to be 0.2 moI L-1, 0.2 mol L-1, and 0.6 moI L-1 respectively.
(a) 48
(b) 9
(c) 0.9
(d) 90
Answer:
(b) 9
Solution:
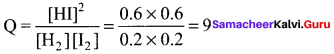
Question 14.
For the reaction N2O4(g) \(\rightleftharpoons\) 2NO2(g) KC = 0.21 at 373 K. The concentrations of N2O4 and NO2 are found to be 0.125 mol dm-3 and 0.5 mol dm-3 respectively at a given temperature. Predict the direction of the reaction.
(a) At equilibrium
(b) reverse direction
(c) forward direction
(d) Both reverse and forward direction
Answer:
(b) reverse direction
Solution:
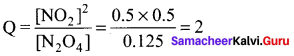
KC = 0.21
Q = 2
Q > KC
Hence the reaction will proced in the reverse direction
Question 15.
Which of the following does not alter the equilibrium?
(a) catalyst
(b) concentration
(c) temperature
(d) pressure
Answer:
(a) catalyst
Question 16.
Statement I. In Haber’s process, NH3 is liquefied and removed.
Statement II. In manufacture of NH3, liquefied and removal of NH3, keeps the reaction moving in forward direction.
(a) Statement I and II are correct and II is the correct explanation of I.
(b) Statement I and II are correct but II is not the correct explanation of I.
(c) Statement I is wrong but statement II is correct.
(d) Statement I is correct but statement II is wrong.
Answer:
(a) Statement I and II are correct and II is the correct explanation of I.
Solution:
Removal of NH3 will decrease its concentration which favours the production of NH3 according to the Le – Chatelier’s principle.
Question 17.
In which of the following reaction, pressure has no effect?
(a) N2 + 3N2 \(\rightleftharpoons\) 2NH3(g)
(b) 2SO2(g) + O2(g) \(\rightleftharpoons\) 2SO3(g)
(c) N2O4(g) \(\rightleftharpoons\) 2NO2(g)
(d) H2(g) + I2(g) \(\rightleftharpoons\) 2HI(g)
Answer:
(d) H2(g) + I2(g) \(\rightleftharpoons\) 2HI(g)
Solution:
In the reaction H2(g) + I2(g) \(\rightleftharpoons\) 2HI(g) the volumes are equal on both sides and so pressure has no effect.
Question 18.
Among the following reactions which one has Kp = KC
(a) N2O4 \(\rightleftharpoons\) 2NO(g)
(b) 2SO2(g) + O2(g) \(\rightleftharpoons\) 2SO3(g)
(c) N2(g) + O2(g) \(\rightleftharpoons\) 2NO(g)
(d) N2(g) + 3H2(g) \(\rightleftharpoons\) 2NH3(g)
Answer:
(c) N2(g) + O2(g) \(\rightleftharpoons\) 2NO(g)
Solution:
Kp = KC. RTΔng
In equation N2(g) + O2(g) \(\rightleftharpoons\) 2NO(g)
Δng = 0
Kp = KC. RT°
Kp = KC
Question 19.
Statement I. Addition of an inert gas at constant volume has no effect on equilibrium.
Statement II. When an inert gas is added, the total number of moles of gases present in the container increases and total pressure also increases, the partial pressure of the products and reactants are unchanged.
(a) Statement I and II are correct but statement II is not the correct explanation of I.
(b) Statement I and II are correct and statement II is the correct explanation of 1.
(c) Statement I is correct but statement II is not correct.
(d) Statement I is wrong but statement II is correct.
Answer:
(b) Statement I and II are correct and statement II is the correct explanation of I.
Question 20.
Which one of the following equation is not correct?
(a) ΔG° = – RTInK
(b) ΔG° = ΔH° – TΔS°
(c) – RTInK = ΔH° – TΔS°
(d) In k = \(\frac { ΔH° }{ T }\) – \(\frac { ΔS° }{ R }\)
Answer:
(d) In k = \(\frac { ΔH° }{ T }\) – \(\frac { ΔS° }{ R }\)
Question 21.
The equilibrium expression, KC = [CO2] represents the reaction.
(a) C(s) + O2(g) \(\rightleftharpoons\) CO2(g)
(b) CaCO3(s) \(\rightleftharpoons\) CaO(s) + CO2(g)
(c) 2CO(g) + O2(g) \(\rightleftharpoons\) 2CO2(g)
(d) CaO(s) + CO2(g) \(\rightleftharpoons\) CaCO3(s)
Answer:
(b) CaCO3(s) \(\rightleftharpoons\) CaO(s) + CO2(g)
Question 22.
Hydrogen molecule (H2) can be dissociated into hydrogen atoms (H). Which one of the following changes will not increase the number of atoms present at equilibrium?
(a) adding H atoms
(b) increasing the temperature
(c) increasing the total pressure
(d) increasing the volume of the container
Answer:
(d) increasing the total pressure container
Solution:
It favours backward reaction i.e., formation of H2 molecule.
Question 23.
What is the expression for Keq for the reaction, 2N2O(g) + O2(g) \(\rightleftharpoons\) 4NO(g)?
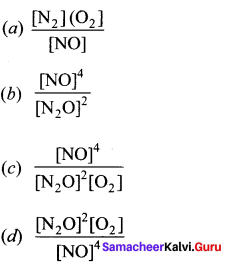
Answer:
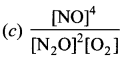
Question 24.
What is the correct expression for the representation of the solubility product constant of Ag2CrO4 ?
(a) [Ag+]2 [CrO42-]
(b) [2Ag+] [CrO42-]
(c) [Ag+] [CrO42-]
(d) [2Ag+]2 [CrO42-]
Answer:
(a) [Ag+]2 [CrO42-]
Question 25.
H2 + S \(\rightleftharpoons\) H2S + energy. In this reversible reaction, select the factor which will shift the equilibrium to the right.
(a) adding heat
(b) adding H2S
(c) blocking hydrogen gas reaction
(d) removing hydrogen suiphide gas
Answer:
(a) removing hydrogen suiphide gas
Question 26.
What effect does a catalyst have on the equilibrium position of a reaction?
(a) a catalyst favours the formation of products
(b) a catalyst favours the formation of reactants
(c) a catalyst does not change the equilibrium position of a reaction
(d) a catalyst may favour reactants or product formation, depending upon the direction in which the reaction is written.
Answer:
(c) a catalyst does not change the equilibrium position of a reaction
Question 27.
A chemist dissolves an excess of BaSO4 in pure water at 25°C if its Ksp = 1 x 10-10. What is the concentration of barium in the water?
(a) 10-4 M
(b) 10-5 M
(c) 10-15 M
(a) 10-6 M
Answer:
(c) 10-15 M
Solution:
Ksp = [Ba2+] [SO42-]
1 x 10-10 = (x) (x)
10-5 = x
Question 28.
If in a mixture where Q = K, then what happens?
(a) the reaction shift towards products
(b) the reaction shift towards reactants
(c) nothing appears to happen, but forward and reverse reactions are continuing at the same rate
(d) nothing happens
Answer:
(c) nothing appears to happen, but forward and reverse reactions are continuing at the same rate
Question 29.
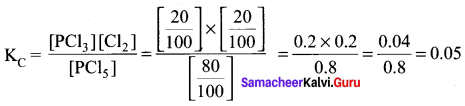
If dissociation for reaction PCI5 \(\rightleftharpoons\) PCI3 + Cl2 is 20% at 1 atm pressure. Calculate the value of KC.
(a) 0.04
(b) 0.05
(c) 0.07
(d) 0.06
Answer:
(d) 0.05
Solution:
Question 30.
What would be the value of Δng for the reaction NH4CI(s) \(\rightleftharpoons\) NH2(g) + HCI(g)?
(a) 1
(b) 0.5
(c) 2
(d) 1.5
Answer:
(c) 2
Solution:
Δng = np – nr = 2 – 0 = 2
Question 31.
Which of the following is not a general characteristic of equilibria involving physical processes?
(a) Equilibrium is possible only in a close system at a given temperature
(b) All measurable properties of the system remain constant
(c) All the physical processes stop at equilibrium
(d) The opposing processes occur at the same rate and there is dynamic but stable condition
Answer:
(c) All the physical processes stop at equilibrium
Question 32.
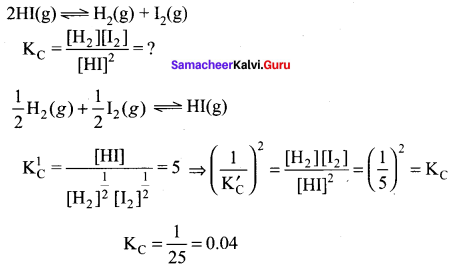
At 500K, equilibrium constant KC for the following reaction is 5, \(\frac { 1 }{ 2 }\)H2(g) + \(\frac { 1 }{ 2 }\)I2(g) \(\rightleftharpoons\) HI(g) what would be the equilibrium constant KC. for the reaction 2HI(g) \(\rightleftharpoons\) H2(g) + I2(g)
(a) 0.44
(b) 0.04
(c) 25
(d) 2.5
Answer:
(b) 0.04
Solution:
Question 33.
For the reaction 2NO2(g) \(\rightleftharpoons\) 2NO(g) + O2 (g), KC = 1.8 x 10-6 at 1850C. At the same temperature the value of KC for the reaction. NO(g) + \(\frac { 1 }{ 2 }\)O2 \(\rightleftharpoons\) NO2(g) is ……….
(a) 0.9 x 106
(b) 7.5 x 102
(c) 1.95 x 10-3
(d) 1.95 x 103
Answer:
(b) 7.5 x 102
Solution:
The reaction is reversed and halved.
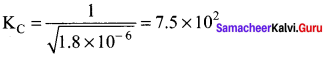
Question 34.
Which of the following reaction will be favoured at low pressure?
(a) N2 + O2 \(\rightleftharpoons\) 2NO
(b) H2 ± I2 \(\rightleftharpoons\) 2HI
(c) PCI5 \(\rightleftharpoons\) PCI3 + Cl2
(d) N2 + 3H2 \(\rightleftharpoons\) 2NH3
Answer:
(c) PCI5 \(\rightleftharpoons\) PCI3 + Cl2
Question 35.
Consider the reaction CaCO3(s) \(\rightleftharpoons\) CaO(s) + CO2(g) is a closed container at equilibrium. What would be the effect of addition of CaCO3 on the equilibrium?
(a) increases
(b) remains unaffected
(c) decreases
(d) unpredictable
Answer:
(b) remains unaffected
Question 36.
For the reaction PCI5(g) \(\rightleftharpoons\) PCI3(g) + Cl2(g) the forward reaction at constant temperature is favoured by …………………
(a) introducing an inert gas at constant volume
(b) introducing PCl3(g) at constant volume
(c) introducing PCl5(g) at constant volume
(d) introducing CI2(g) at constant volume
Answer:
(c) introducing PCI5(g) at constant volume
Question 37.
The equilibrium of the reaction N2(g) + 3H2(g) \(\rightleftharpoons\) 2NH3(g) will shift to product side when….
(a) Kp > 1
(b) Q < Kp
(c) Q = Kp
(d) Q = 2Kp
Answer:
(b) Q < Kp
Question 38.
NO2 is involved in the formation of smog and acid rain. A reaction that is important in the formation of NO2 is O3(g) + NO(g) \(\rightleftharpoons\) O2(g) + NO2(g) KC = 6.0 x 1034. If the air over a section of New Delhi contained 1.0 x 10-6 M of O3, 1.0 x 10-5 M of NO, 2.5 x 10-4 M of NO2 and 8.2 x 10-3 of O2, what can we conclude?
(a) there will be a tendency to form more NO and O2
(b) there will be a tendency to form more NO2 and O2
(c) there will be a tendency to form more NO2 and O3
(d) there will no tendency for change because the reaction is at equilibrium
Answer:
(b) there will be a tendency to form more NO2 and O2
Solution:
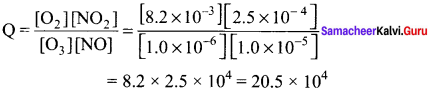
As Q < KC the reaction will hive a tendency to move forward.
Question 39.
Haemoglobin (Hb) forms bond with oxygen and given oxyhaemoglobin (HbO2). This process is partially regulated by the concentration of H3O+ and dissolved CO2 in blood as HbO2 +H3O+ + CO2 \(\rightleftharpoons\) H+ – Hb – CO2 + O2 + H2O. If there is production of lactic acid and CO2 during a muscular exercise, then
(a) more HbO2 is formed
(b) more O2 is released
(c) CO2 is released
(d) both (b) and (c)
Answer:
(b) more O2 is released
Question 40.
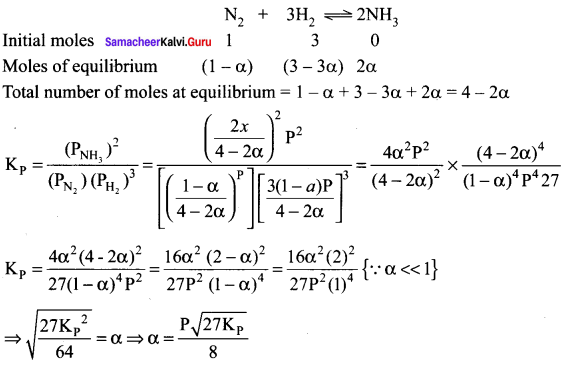
In the reaction N2 + 3H2 \(\rightleftharpoons\) 2NH3 + x k Cal, one mole of N2 reacts with. 3 moles of H2 at equilibrium. Then the value of a (degree of dissociation) is approximately ……………… P is the pressure at equilibrium
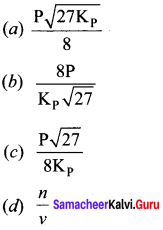
Answer:
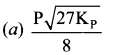
Solution:
II. Match the following.
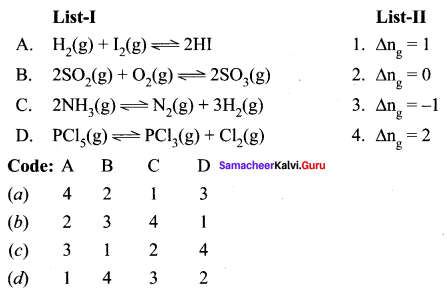
Question 1.
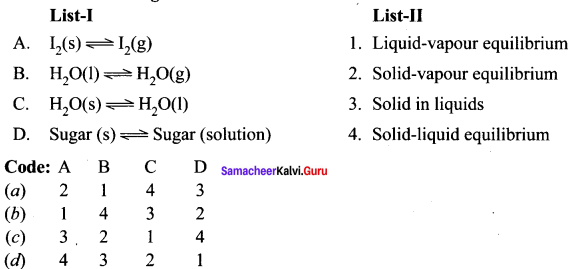
Answer:
(a) 2, 1, 4, 3
Question 2.
Answer:
(b) 2, 3, 4, 1
Question 3.
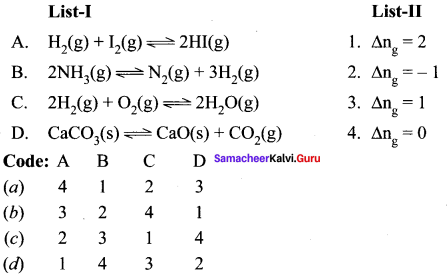
Answer:
(a) 4, 1, 2, 3
Question 4.
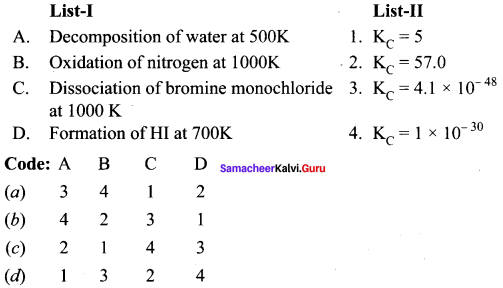
Answer:
(a) 3, 4, 1, 2
Question 5.
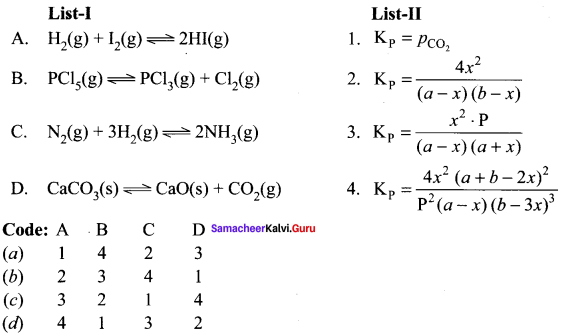
Answer:
(b) 2, 3, 4, 1
III. Fill in the blanks.
Question 1.
Transport of oxygen by Hemoglobin in our body is ………… a reaction.
Answer:
reversible
Question 2.
The temperature at which the solid and liquid phases of a substance are at equilibrium is called …………
Answer:
Freezing point
Question 3.
Thejemperature at which the liquid and vapour phases are at equilibrium is called …………
Answer:
Condensation point
Question 4.
………… law is used to explain gas-solution equilibrium processes.
Answer:
Henry’ law
Question 5.
In the reaction 2H2(g) + O2(g) \(\rightleftharpoons\) 2H2O(g), the Kp value is equal to ………
Answer:
< KC
Solution:
Δng = 2 – 3 = -1
![]()
Qustion 6.
The expression of K for the reaction CO2(g) + H2O(l) \(\rightleftharpoons\) H+(aq) + HCO3–(aq) is equal to …………..
Answer:
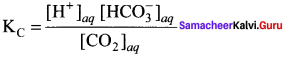
Question 7.
The expression of K for the reversible reaction 2CO(g) \(\rightleftharpoons\) CO2(g) + C(s)
Answer:
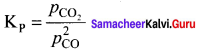
Question 8.
The Ang value for the reaction 2NO(g) + O2(g) \(\rightleftharpoons\) 2NO2(g) is …………….
Answer:
– 1
Solution:
Δng = np – nr = 2 – 3 = -1
Question 9.
The correct differential form of van’t Hoff equation is ………….
Answer:
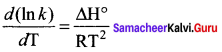
Question 10.
For the reaction H2(g) + I2(g) \(\rightleftharpoons\) 2HI(g), the equilibrium constant K is
Answer:

Question 11.
PCI5 is kept in a closed container at a temperature of 250K the equilibrium concentrations of PCI5, PCl3 and Cl2 are 0.045 moles L-1, 0.096 moles L-1, 0.096 moles L-1 respectively. The value of equilibrium constant for the reaction PCI5(g) \(\rightleftharpoons\) PCl3(g) + Cl2(g) will be ……………………
Answer:
0.205
Solution:
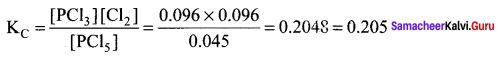
Question 12.
Equilibrium constant changes with ………………..
Answer:
Both temperature and pressure
Question 13.
For the reaction 2HI(g) \(\rightleftharpoons\) H2(g) + I2(g) at 720 K, the equilibrium constant value is 50. The equilibrium constant for the reaction H2(g) + I2(g) \(\rightleftharpoons\) 2HI(g) at the same temperature will be ………..
Answer:
0.02
Solution:
Forward reaction equilibrium constant K1 = 50
Reverse reaction equilibrium constant K2 = ?
K2 = \(\frac { 1 }{ { K }_{ 1 } }\) = \(\frac { 1 }{ 50 }\) = 0.02
Question 14.
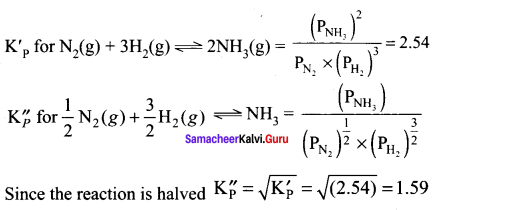
If equilibrium constant for the reaction N2(g) + 3H2(g) \(\rightleftharpoons\) 2NH3(g) at 298 K is 2.54, the value of equilibrium constant for the reaction \(\frac { 1 }{ 2 }\)N2 + \(\frac { 3 }{ 2 }\)H2 \(\rightleftharpoons\) NH3 will be ………….
Answer:
1.59
Solution:
Question 15.
The chemical system at equilibrium is not affected by addition of ……………..
Answer:
catalyst
Question 16.
A catalyst will increase the rate of a chemical reaction by lowering the ……………
Answer:
activation energy
Question 17.
In a closed system, A(s) \(\rightleftharpoons\) 3B(g) + 3C(g) If partial pressure of C is doubled, then partial pressure of B will be ………….. times the original value.
Answer:
\(\frac { 1 }{ 2\sqrt { 2 } }\)
Question 18.
Consider the following gaseous equilibria with equilibrium constants K1 and K2 respectively
SO2 + \(\frac { 1 }{ 2 }\)O2(g) \(\rightleftharpoons\) SO3(g) – K1
2SO3(g) \(\rightleftharpoons\) 2SO2(g) + O2(g) – K2
The equilibrium constants are related as ………….
Answer:
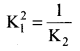
Solution:

Question 19.
K2 for the following reaction at 700 K is 1.3 x 10-3 atm-1. The K at same temperature for the reaction 2SO2(g) + O2(g) \(\rightleftharpoons\) 2SO3(g) will be ………….
Answer:
7.4 x 10-2
Solution:
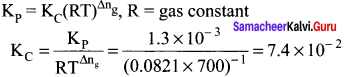
Question 20.
For the reaction PCI23(g) + CI2(g) \(\rightleftharpoons\) PCI5(g) at 250°C, the value of KC is 26 then the value of Kp on the same temperature will be ……………
Answer:
0.61
Solution:
![]()
Question 21.
In the reaction N2(g) + 3H2(g) \(\rightleftharpoons\) 2NH3(g), the value of the equilibrium constant depends on ………………
Answer:
the temperature
Question 22.
K1 and K2 are velocity constant o forward and backward reactions. The equilibrium constant KC of the reaction is …………
Answer:
\(\frac { { K }_{ 1 } }{ { K }_{ 2 } }\)
Question 23.
The equilibrium constant of the reaction 3C2H2 \(\rightleftharpoons\) C6H6 is 4.0 at a temperature of T K. If the equilibrium concentration of C2H2 is 0.5 molesL-1, the concentration of C6H6 is …………..
Answer:

Question 24.
In an equilibrium reaction for which ΔG0 = 0, the equilibrium constant K should be = ……………
Answer:
1
Question 25.
The equilibrium constant for the reaction 2SO2(g) + O2(g) \(\rightleftharpoons\) 2SO3(g) is 5. If the equilibrium constant mixture contains equal moles of SO3 and SO2, the equilibrium partial pressure of O2,
gas is ……………..
Answer:
0.2 atm
Solution:
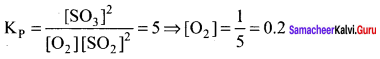
Question 26.
In the reaction NH4CI(s) \(\rightleftharpoons\) NH3(g) + HCI(g) the value Offlg is Δng ………………….
Answer:
2
Solution:
Δng = np(g) – nr(g) = 2 – 0 = 2
IV. Choose the odd one out.
Question 1.
(a) see – saw
(b) tug – of – war
(c) sublimation of camphor
(d) Acid hydrolysis of an ester
Answer:
(d) Acid hydrolysis of an ester Acid hydrolysis of an ester is a chemical equilibrium.
![]()
Whereas a, b, c are examples of physical equilibrium.
Question 2.
(a) Synthesis of hydrogen iodide
(b) Decomposition of calcium carbonate
(c) Sublimation of iodine
(d) Dissociation of PCl5
Answer:
(c) Sublimation of iodine Sublimation of iodine I2(s) \(\rightleftharpoons\) I2(g) is an example of physical equilibrium, whereas a, b and dare examples of chemical equilibrium.
Question 3.
(a) Synthesis of HI
(b) Dissociation of PCl5
(e) Synthesis of NH3
(d) Decomposition of CaCO3
Answer:
(a) Decomposition of CaCO3
Decomposition of CaCO3 is an example of heterogeneous equilibrium.
CaCO3(s) \(\rightleftharpoons\) CaO(s) + CO2(g)
Where as a, b, c are examples of homogeneous equilibrium.
Question 4.
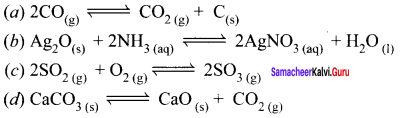
Answer:
![]()
Synthesis of SO3 is an example of homogeneous equilibrium whereas the others a, b and d are heterogeneous equilibrium.
Question 5.
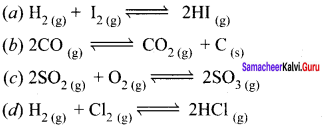
Answer:
![]()
(b) is an example of heterogeneous equilibrium whereas the others a, c and d are homogeneous equilibrium.
V. Choose the correct pair.
Question 1.
(a) Q = KC : Reaction is in equilibrium state
(b) Q < KC : Reaction proceed in reverse direction
(c) Q > KC : Reaction proceed in ftrward direction
(d) Q = KC : Reaction proceed in both directions
Answer:
(a) Q = KC : Reaction is in equilibrium state
Question 2.
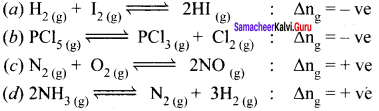
Answer:
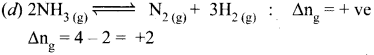
Question 3.
(a) Kp = KC : Synthesis of HI
(b) Kp > KC : Dissociation of PCl5
(c) Kp < KC : Synthesis of SO3
(d) Kp = KC : Synthesis of HI
Answer:
(a) Kp = KC : Synthesis of HI
H2(g) + I2(g) \(\rightleftharpoons\) 2HI(g) : Δng = 2 – 2 = 0
ΔKp = KC
VI. Choose the incorrect pair.
Question 1.
(a) Acid hydrolysis of an ester – Homogeneous equilibrium
(b) Synthesis of Ammonia – Homogeneous equilibrium
(c) Decomposition of CaCO3 – Homogeneous equilibrium
(d) Synthesis of HI – Homogeneous equilibrium
Ans.
(c) Decomposition of CaCO3: Homogeneous equilibrium It is a heterogeneous equilibrium.
Question 2.
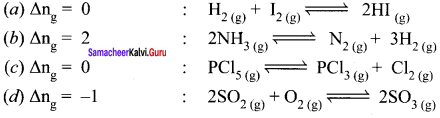
Answer:

VII. Assertion and Reason.
Question 1.
Assertion (A): Chemical equilibrium is in a state of dynamic equilibrium.
Reason (R): At equilibrium the forward and backward reactions are proceeding at the same rate and no macroscopic change is observed.
(a) Both (A) and (R) are correct and (R) is the correct explanation of(A).
(b) Both (A) and (R) are correct but (R) is not the correct explanation of(A).
(c) (A) is correct but (R) is wrong.
(d) (A) is wrong but (R) is correct.
Answer:
(a) Both (A) and (R) are correct and (R) is the correct explanation of(A).
Question 2.
Assertion (A): In Haber’s process, NH3 is liquefied and removed.
Reason (R): Because of the reaction keeps moving in the backward direction.
(a) Both (A) and (R) are correct but (R) is not the correct explanation of(A).
(b) Both (A) and (R) are correct and (R) is the correct explanation of(A).
(c) (A) is correct but (R) is wrong.
(d) (A) is wrong but (R) is correct.
Answer:
(b) Both (A) and (R) are correct and (R) is the correct explanation of(A).
Question 3.
Assertion (A): In the dissociation of PCI5 at constant pressure and temperature addition of helium at equilibrium increases the dissociation of PCl5.
Reason (R): Helium removes CI, from the field of action.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is the not the correct explanation of A.
(c) A is true but R is false
(d) Both A and R are false
Answer:
(d) Both A and R are false
VIII. Choose the incorrect statement.
Question 1.
(a) In equilibrium mixture of ice and water kept in perfectly insulted flask, mass of ice and water does not change with time.
(b) The intensity of red colour increases when oxalic acid is added to a solution containing iron (III) nitrate and potassium thiocyanate
(c) On addition of catalyst the equilibrium constant value is not affected
(d) Equilibrium constant for a reaction with negative .H value decreases as the temperature increases.
Answer:
(b) The intensity of red colour increases when oxalic acid is added to a solution containing iron (III) nitrate and potassium thiocyanate
Solution:
Oxalate ions of oxalic acid form complex with ferric ions thus decrease its concentration thus, concentration of red complex in product decreases.
Samacheer Kalvi 11th Chemistry Physical and Chemical Equilibrium 2 Mark Questions and Answers.
I. Write brief answer to the following questions:
Question 1.
Define the state of equilibrium.
Answer:
At a particular stage, the rate of the reverse reaction is equal to that of the forward reaction indicating a state of equilibrium.
Question 2.
What are the different types of equilibrium? Explain with example?
Answer:
1. Physical equilibrium:
A system in which the amount of matter constituting different phases does not change with time is said to be in physical equilibrium.
H2O(s) \(\rightleftharpoons\) H2O(1). Solid-liquid equilibrium.
2. Chemical equilibrium:
Chemical reactions in which the forward and backward reactions are proceeding at the same rate and no macroscopic change is observed is said to be in chemical equilibrium. H2(g) + I2(g) \(\rightleftharpoons\) 2HI(g)
Question 3.
Explain about the equilibrium involving dissolution of solid in liquid with suitable example.
Answer:
When sugar is added to water at a particular temperature. it dissolves to form sugar solution. When more sugar is added to that solution, a particular stage sugar remains as solid and results in the formation of saturated solution. Here a dynamic equilibrium is established between the solute molecules ii the solid phase and in the solution phase.
![]()
Question 4.
How is a gas – solution equilibrium exist?
Answer:
When a gas dissolves in a liquid under a given pressure, there will be an equilibrium between gas molecuLes in the gaseous state and those dissolved in the liquid. Example – In carbonate beverages the following equilibrium exists.
CO2(g) \(\rightleftharpoons\) CO2 (Solution)
Question 5.
What is meant by active mass? Give its unit.
Answer:
The active mass represents the molar concentration of the reactants (or) products.
![]()
Question 6.
Show that Kp = KC with two example
Answer:
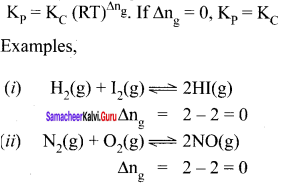
Question 7.
Give two examples of equilibrium reactions where Kp > KC.
Answer:
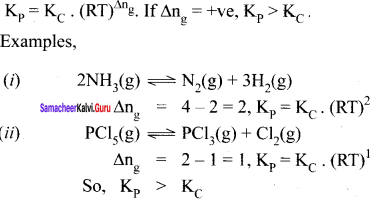
Question 8.
When will be Kp < KC? Give two example.
Answer:
Question 9.
Write the KC for the reaction CO2(g) + H2O(l) \(\rightleftharpoons\) H+(aq) + HCO3(aq)–
Answer:
H2O(l) is a pure liquid and its concentration remains constant.
Solution:
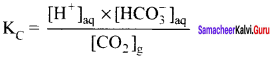
Question 10.
![]() What is the value of K4 in A \(\rightleftharpoons\) D
What is the value of K4 in A \(\rightleftharpoons\) D
Answer:
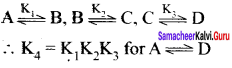
Question 11.
Write the Kp and KC for the following reactions
(i) 2SO2(g) + O2(g) \(\rightleftharpoons\) 2SO3(g)
(ii)2CO(g) \(\rightleftharpoons\) CO2(g) + C(s)
Answer:
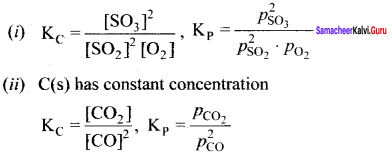
Question 12.
Explain how the equilibrium constant K. predict the extent of a reaction.
Answer:
1. The value of equilibrium constant KC tells us the extent of the reaction i.e., it indicates how far the reaction has proceeded towards product formation at a given temperature.
2. A large value of KC indicates that the reaction reaches equilibrium with high product yield on the other hand, lower value of KC indicates that the reaction reaches equilibrium with low product yield.
3. If KC > 103 the reaction proceeds nearly to completion.
4. If KC < 10-3 the reaction rarely proceeds.
5. If the KC is in the range 10-3 to 103 significant amount of both reactants and products are present at equilibrium.
Question 13.
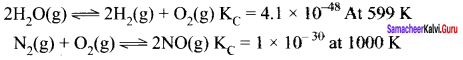
Predict the extent of the above two reactions.
Answer:
In the reactions, decomposition of water at 500 K and oxidation 01 nitrogen at 1000 K, the value of KC is very less KC < 10-3. So reverse reaction is favoured.
∴ Products << reactants
Question 14.
Explain about the extent of reaction of dissociation of bromine mono chloride at 1000 K.
Answer:
2BrCl(g) \(\rightleftharpoons\) Br2(g) + Cl2(g) KC = 5
10-3 < KC < 103
both forward and backward reaction make significant progress. Neither forward nor reverse reaction predominates.
Question 15.
What is the KC value for formation of HCl at 700 K? Predict the extent of the reaction?
Answer:
H2(g) + I2(g) \(\rightleftharpoons\) 2HI(g) at 700 K
KC = 57.0
10-3 <KC <103
So both forward and backward reaction make significant progress. Neither forward nor reverse reaction predominates.
Question 16.
What is the KC value of formation of HCI at 300 K? Explain it.
Answer:
H2(g) – Cl2(g) \(\rightleftharpoons\) 2HCI(g) at 300 K
K2 = 4 x 1031
KC > 103 So[products] >> [Reactant]
Reaction nearly goes to completion. So forward reaction is favoured.
Question 17.
2CO(g) + O2(g) \(\rightleftharpoons\) 2CO2(g) at 1000 K. What is the Kc this reaction? Predict the extent of this reaction.
Answer:
2CO(g) + O2(g) \(\rightleftharpoons\) 2CO2(g) at 1000 K
KC = 2.2 x 1022
KC > 103.
So [Products] >> [Reactants]
Reaction nearly goes to completion and forward reaction is favoured.
Question 18.
Define Q value for a chemical equilibrium reaction.
Answer:
Consider a homogeneous reversible reaction xA + yB \(\rightleftharpoons\) lC + mD For the above reaction under non-equilibrium conditions, reaction quotient Q is defined as the ratio of the product of active masses of reaction products raised to the respective stoichiometric coefficients in the balanced chemical equation to that of the reactants. Under non equilibrium conditions,
Q = \(\frac { d(Ink) }{ dt }\) ΔH° = \(\frac { { \triangle H }^{ 0 } }{ { RT }^{ 2 } }\)
Question 19.
Explain the diagrammatic expression about the direction of reaction.
Answer:
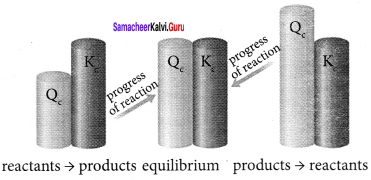
In (i) Q < KC, the reaction will proceed in forward direction.
In (ii) Q = KC, the reaction is in equilibrium state.
In (iii) Q> KC, the reaction will proceed in the reverse direction.
Question 20.
Explain about the effect of catalyst in an equilibrium reaction?
Answer:
Addition of catalyst does not affect the state of equilibrium. The catalyst increases the rate of both the forward and reverse reactions to the same extent. Hence it does not change the equilibrium composition of the reaction mixture.
Question 21.
For the following equilibrium. KC = 6.3 x 1014 at 1000K
NO(g) + O3(g) \(\rightleftharpoons\) NO2(g) + O2(g)
Both the forward and reverse reactions in the equilibrium are elementary bimolecular reactions what is KC for the reverse reaction?
Answer:
For the reverse reaction 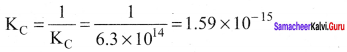
Question 22.
Explain why pure liquids and solids can be ignored while writing the value of equilibrium constants.
Answer:
This is because molar concentration of a pure solid or liquid is independent of the amount present.
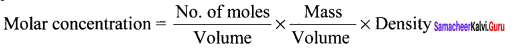
Since density of pure liquid or solid is fixed and molar mass is also fixed, therefore molar concentration are constant.
Question 23.
A sample of Hl(g) is placed in a flask at a pressure of 0.2 atm. At equilibrium partial pressure of HI(g) is 0.04 atm. What is Kp for the given equilibrium?
2HI(g) \(\rightleftharpoons\) H2 (g) + I2(g)
Answer:
pHI = 0.04 atm. pH2 = 0.08 atm; pl2 = 0.08 atm
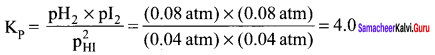
Question 24.
The equilibrium constant expression for a gas reaction is.
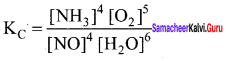
Write the balanced chemical equation corresponding to this expression.
Answer:
Balanced chemical equation for the reaction is
4 NO(g) + 6 H2O(g) \(\rightleftharpoons\) 4NH3(g) + 5O2(g)
Question 25.
Predict which of the following will have appreciable concentration of reactions and products:
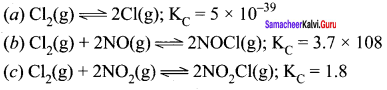
Answer:
Following conclusion can be drawn from the values of
(a) Since the value of K is very small, this means that the molar concentration of the products is very small as compared to that of the reactants.
(b) Since the value of K is quite large, this means that the molar concentration of the products is very large as compared to that of the reactants.
(c) Since the value of K is 1.8, this means that both the products and reactants have appreciable concentrations.
Question 26.
Write the equilibrium constant (KC) expression for the following reactions.
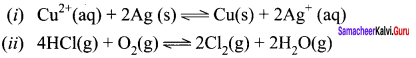
Answer:

Question 27.
For the equilibrium 2NOCI(g) \(\rightleftharpoons\) 2NO(g) + CI2(g) the value of the equilibrium constant KC is 3.75 x at 1069 K. Calculate the Kp for the reaction at this temperature?
Answer:
We know that Kp = Kc(RT)∆ng
For the above reaction, ∆ng = (2 + 1) – 2 = 1
Kp = 3.75 x 10-6 (0.0831 x 1069) = 3.3 x 10-4
Question 28.
The value of Kc for the reaction 2A \(\rightleftharpoons\) B + C is 2 x 10-3. At a given time, the composition of reaction mixture is [A] [B] [C] = 3 x 10-4 M. In which direction the reaction will proceed?
Answer:
For the reaction, the reaction quotient Q is given by QC = [B] [C]/[A]2
as [A] = [B] = [C] = 3 x 10-4 M
Qc (3 x 10-4)(3 x 10-4)/(3 x 10-4)2 = l
as QC > KC so, the reaction will proceed in the reverse direction.
Question 29.
Dihydrogen gas is obtained from natural gas by partial oxidation with steam as per following endothermic reaction:
CH4(g) + H2O(g) \(\rightleftharpoons\) CO(g) + 3H2(g)
(a) Write an expression for Kp for the above reaction.
(b) How will the values of Kp and composition of equilibrium mixture be affected by
(i) increasing the pressure
(ii) increasing the temperature
(iii) using a catalyst
Answer:
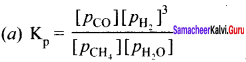
(b) (I) value of Kp will not change, equilibrium will shift in backward direction.
(ii) value of Kp will increase and reaction will proceed in forward direction.
(iii) no effect.
II. Answer the following questions:
Question 1.
Explain about the formation of solid-liquid equilibrium with suitable example.
Answer:
1. Consider melting of ice in a closed container at 273 K. This system reach a state of physical equilibrium in which the amount of water in the solid phase and liquid phase does not change with time.
2. In this process. the total number of water molecules leaving from and returning to the solid phase at any instant are equal.
3. If some ice cubes and water are placed in a thermos flask (at 273K and I atm) then there will be no change in the mass of ice and water.
4. At equilibrium: Rate of melting of ice Rate of freezing of water
H2O(l) \(\rightleftharpoons\) H2O(g)
Question 2.
How is liquid – vapour equilibrium exist?
Answer:
1. Liquid water is in equilibrium with vapour at 373 K and I atm pressure in a closed vessel
2. Rate of evaporation = Rate of condensation
Question 3.
What is meant by boiling point and condensation point of the liquid?
Answer:
The temperature at which the liquid and vapour phases are at equilibrium is called the boiling point and condensation point of the liquid.
Question 4.
Define melting point (or) freezing point of the substance.
Answer:
The temperature at which the solid and liquid phases of a substance are at equilibrium is called the melting point or freezing point of substance.
Question 5.
Illustrate the formation of solid – vapour equilibrium with suitable example.
Answer:
1. Consider a system in which the solid sublimes to vapour. e.g., I, (or) camphor.
2. When solid iodine is placed in a closed transparent vessel, after sometime, the vessel gets filled up with violet vapour due to sublimation of iodine.
3. Initially the intensity of the violet colour increases, after some time it decreases and finally it becomes constant as the following equilibrium is attained.
I2(s) \(\rightleftharpoons\) I2 (g)
Question 6.
Give three examples for solid vapour equilibrium.
Answer:
I2(s) \(\rightleftharpoons\) I2 (g)
Camphor (s) \(\rightleftharpoons\) Camphor (g)
NH4CI(s) \(\rightleftharpoons\) NH4CI(g)
Question 7.
Explain the following diagrams.
Diagram – 1
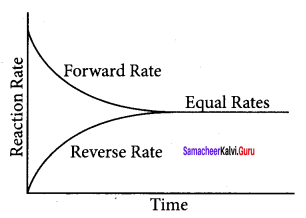
Answer:
- As the concentration of the products increases, more products collide and react in the backward direction.
- As the rate of the reverse reaction increases, the rate of the forward reaction decreases.
- Eventually the rate of both reactions becomes equal.
Diagram-II
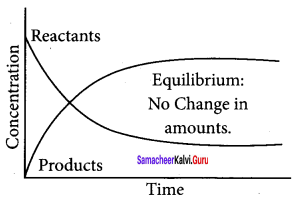
- Concentration of reactants decreases with time initially and concentration of products increases with time.
- After sometime, equilibrium is reached i.e., concentration of reactants and products remains constant.
Question 8.
What are the types of chemical equilibrium? Explain with suitable example.
Answer:
- Chemical equilibrium is of two types:
- Homogeneous equilibrium
- Heterogeneous equilibrium.
- In a homogeneous equilibrium, all the reactants and products are in the same phase
H2(g) + I2(g) \(\rightleftharpoons\) 2HI(g)
In the above equilibrium H2, I2 and HI are in the gaseous state. - If the reactants and products of a reaction in equilibrium are in different phases, then it is calLed heterogeneous equilibrium.
e.g., CaCO3(s) \(\rightleftharpoons\) CaO(s) + CO3(g)
Question 9.
Write the value of K and K equation for CaCO3(s) \(\rightleftharpoons\) CaO(s) + CO2(g)
Answer:
A pure solid always has the same concentration at a given temperature, as it does not expand to fill its container i.e., it has the same no. of moles of its volume. Therefore the concentration of pure solid is a constant. So the expression if K and K is K [CO2], K = PCO2
Question 10.
Consider the following equilibrium reaction and relate their equilibrium constants
- N2 + O2 \(\rightleftharpoons\) 2NO, K1
- 2NO + O2 \(\rightleftharpoons\) 2NO2, K1
- N2 + 2O2 \(\rightleftharpoons\) 2NO2, K3
Answer:
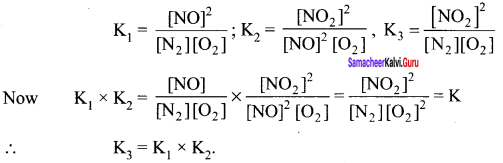
Question 11.
Explain the effect of concentration in an equilibrium state?
Answer:
At equilibrium, the concentration of the reactants and products does not change. The addition of more reactants or products to the reacting system at equilibrium causes an increase in their respective concentration.
According to Le – Chatelier’s principle, the effect of increase in concentration of a substance is to shift the equilibrium in a direction that consumes the added substance. For example,
H2(g) + I2(g) \(\rightleftharpoons\) 2HI(g)
The addition of H2 or I2 to the equilibrium mixture, disturbs the equilibrium. In order to minimize the stress, the system shifts the reaction in a direction where H2 and I2 are consumed i.e., formation of additional HI would balance the effect of added reactant.
Hence the equilibrium shifts to the right (forward direction). i.e., the equilibrium is re – established. Similarly, removal of HI (Product) also favours forward reaction. If HI is added to the equilibrium mixture, the concentration of HI is increased and system proceeds in the reverse direction to nullify the effect of increase in concentration of HI.
Question 12.
Consider the reaction, N2(g) + 3H2(g) \(\rightleftharpoons\) 2NH3(g). Explain the effect of pressure on this equilibrium reaction.
Answer:
N2(g) + 3H2(g) \(\rightleftharpoons\) 2NH3(g)
In the above equilibrium, if the pressure is increased, the volume wiLl decreases. The system responds to this effect by reducing the number of gas molecules. i.e., it favours the formation of ammonia. If the pressure is reduced, the volume will increases. It favours the decomposition of ammonia.
Question 13.
Why pressure has no effect on the synthesis of HI?
Answer:
(i) When the total number of moles of gaseous reactants and gaseous products are equal, the change in pressure has no effect on system at equilibrium.
H2(g) + I2(g) \(\rightleftharpoons\) 2HI(g)
Here the number of moles of reactants and products are equal. So the pressure has no effect on such equilibrium with ∆ng = O.
Question 14.
Explain the effect of temperature on the following equilibrium reaction.
N2(g) + 3H2(g) \(\rightleftharpoons\) 2NH3(g) ∆H = – 92.2 kJ.
Answer:
In this equilibrium, N2(g) + 3H2(g) \(\rightleftharpoons\) 2NH3(g) ∆H = – 92.2 kJ.
Forward reaction is exothermic while the reverse reaction is endothermic. If the temperature of the system increased, the system responds by decomposing some of ammonia molecules to nitrogen and hydrogen by absorbing the supplied heat energy.
Similarly, the system responds to a drop in the temperature by forming more ammonia molecule from nitrogen and hydrogen which release heat energy.
Question 15.
How does oxygen exchanges between maternal and fetal blood in a pregnant woman?
Answer:
1. In a pregnant women, the oxygen supply for the fetus is provided by the maternal blood in the placenta where the blood vessels of both mother and fetus arc in close proximity. Both fetal and maternal hemoglobin binds tO oxygen reversibly as follows.
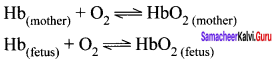
2. In the above two equilibrium, the equilibrium constant value for the oxygenation of fetal hemoglobin is higher which is due to its higher affinity for oxygen compared to adult hemoglobin. Hence in placenta, the oxygen from the mother’s blood s effectively transferred to fetal hemoglobin.
Question 16.
What is K for the following reaction in state of equilibrium?
2SO2(g) + O2 \(\rightleftharpoons\) 2SO3(g)
(Given:[SO2] = 0.6 M; [O2] = 0.82 M; and [SO3] = 1.90 M
Answer:
2SO2(g) + O2 \(\rightleftharpoons\) 2SO3(g)
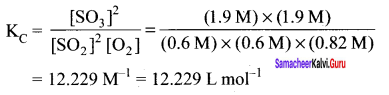
Question 17.
At a certain temperature and total pressure of 105Pa, iodine vapours contain 40% by volume of iodine atoms in the equilibrium 12(g) \(\rightleftharpoons\) 2I(g). Calculate Kp for the equilibrium.
Answer:
According to available data:
Total pressure of equilibrium mixture = 105 Pa
Partial pressure of iodine atoms (1) = \(\frac { 40 }{ 100 }\) x (105 Pa) = 0.4 x 105 Pa
Partial pressure of iodine molecules (I2) = \(\frac { 60 }{ 100 }\) x (105 Pa) = 0.6 X 105 Pa
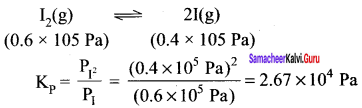
Question 18.
A mixture of 1.57 mol of N2, 1.92 mol of H2 and 8.13 mol of NH3 is introduced into a 20 L reaction vessel at 500 K. At this temperature, the equilibrium constant KC for the reaction
N2(g) + 3H2(g) \(\rightleftharpoons\) 2NH3(g) is 1.7 x 10-2
Is this reaction at equilibrium? if not. what is the direction of net rection?
Answer:
The reaction is: N2(g) + 3H2(g) \(\rightleftharpoons\) 2NH3(g)
Concentration quotient (Q ) =
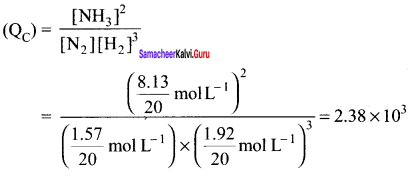
The equilibrium constant (Kr) for the reaction = 1.7 x 10-2
As QC \(\neq\) KC; this means that the reaction is not in a state of equilibrium.
Question 19.
What is the effect of:
- addition of H2
- addition of CH3OH
- removal of CO
- removal of CH3OH
On the equilibrium 2H2(g) + CO(g) \(\rightleftharpoons\) CH3OH(g)
Answer:
- Equilibrium will be shifted in the forward direction.
- Equilibrium will be shifted in the backward direction.
- Equilibrium will be shifted in the backward direction.
- Equilibrium will be shifted in the forward direction.
Question 20.
At 473 K, the equilibrium constant Kc for the decomposition of phosphorus pentachioride (PCl5) is 8.3 x 10-3 . If decomposition proceeds as:
Answer:
PCI5(g) \(\rightleftharpoons\) PCI3(g) + Cl2(g);
∆H = + 124.0 kJ mol-1
- Write an expression for K. for the reaction.
- What is the value of K for the reverse reaction at the same temperature.
- What would be the effect on KC if
- More of PCI5 is added
- Temperature is increased.
Answer:
1. The expression for 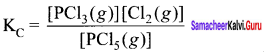
2. For reverse reaction 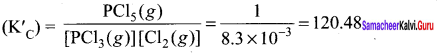
3.
(I) By adding more of PCI5, value of Kc will remain constant because there is no change in temperature.
(ii) By increasing the temperature the forward reaction will be favoured since it is endotherniic in nature. Therefore, the value of equilibrium constant will increase.
Question 21.
Dihydrogen gas used in Haher’s process is produced by reacting methane from natural gas with high temperature stam. The first stage of two stage reaction involves the formation of CO and H2. In second stage, CO formed in first stage is reacted with more steam in water gas shift reaction.
Answer:
CO(g) + H2O(g) \(\rightleftharpoons\) CO2(g) + H2(g)
If a reaction vessel at 400°C is charged with an equimolar mixture of CO and steam so that PCO = PH2O = 4.0 bar., what will be the partial pressure of 2 at equilibrium? Kp = 0.1 at 400°C.
Answer:
Let the partial pressure of hydrogen (H2) at equilibrium point p bar
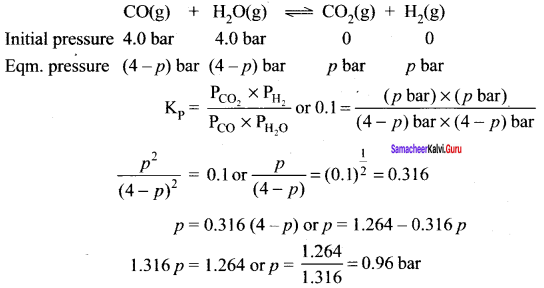
Question 22.
The value of KC for the reaction 3O2(g) \(\rightleftharpoons\) 2O3(g) is 2.0 x 10-50 So at 25°C. If equilibrium concentration of 0, in 25°C is 1.6 x 102 what is the concentration of O3?
Answer:
3O2(g) \(\rightleftharpoons\) 2O3(g)
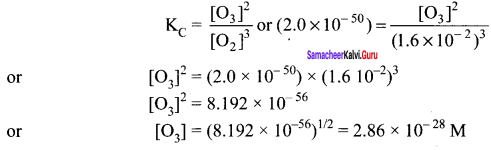
Question 23.
The reaction CO(g) + 3H2(g) \(\rightleftharpoons\) CH4(g) + H2O(g) is at equilibrium at 1300 K in a 1K flask. It also contain 0.30 mol of CO, 0.10 mol of H2 and 0.02 mol of H2O and an unknown amount of CH4 in the flask. Determine the concentration of CH4 in the mixture. The equilibrium constant, KC for the reaction at the given temperature is 3.90.
Answer:
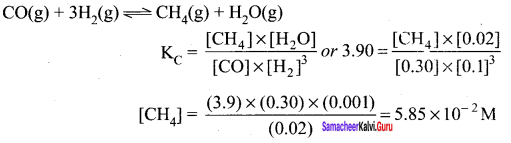
Question 24.
The following concentration were obtained for the formation of NH3 from N2 and H2 at equilibrium at 500 K.
- [N2(g)] 1.5 x 10-2 M
- [H2(g)] = 3.0 x 10-2 M
- [NH3]= 1.2 x 10-2M.
Calculate equilibrium constant.
Answer:
N2(g) + 3H2(g) \(\rightleftharpoons\) 2NH3(g)
Calculate equilibrium constant
Answer:
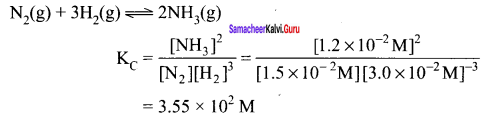
Question 25.
- In the reaction A+ B → C + D, what will happen to the equilibrium if concentration of A is increased?
- The equilibrium constant for a reaction is 2 x 10-23 at 25°C and 2 x 10-2 at 50°C. Is the reaction endothermic or exothermic?
- Mention at least three ways by which the concentration of SO3 can be increased in the following reaction in a state of equilibrium. 2SO2(g) + O2(g) \(\rightleftharpoons\) 2SO3(g)
Answer:
- The reaction will shift in the forward direction.
- Endothermic
- following reaction
- increasing concentration of SO2
- increasing pressure.
- increasing concentration of oxygen.
![]()
Question 26.
PCI5, PCI3 and CI2 are at equilibrium at 500 K and having concentration I.59M PCl3, l.59M
CI2 and 1.41M PCI5. Calculate K. for the reaction PCl5 \(\rightleftharpoons\) PCl3 + Cl2
Answer:
The equilibrium constant K. for the above reaction can be written as:
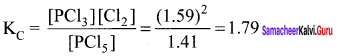
Question 27.
Given the equilibrium
N2O4(g) \(\rightleftharpoons\) 2NO2(g) with Kp = 0.15 atm at 298 K
(a) What is Kp using pressure in torr?
(b) What is KC using units of moles per litre.
Answer:
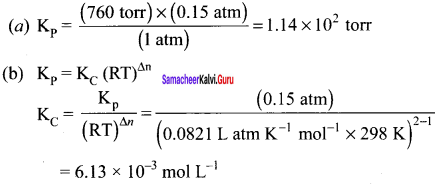
III. Answer the following questions in detail:
1. Derive the values of equilibrium constants Kp and KC for a general reaction
x A + y B \(\rightleftharpoons\) lC + mD
Answer:
Let us consider a reversible reaction x A + y B \(\rightleftharpoons\) lC + mD
where A, B are the reactants C and D are the product and x, y. l and m are the stoichiometric coefficients of A. B, C and D respectively. Applying the law of mass action the rate of forward reaction.
rf ∝ [A]x [B]y or rf Kf [A]x [B]y
Similarly the rate of backward reaction
rb ∝ [C]l [D]m or rb = Kb [C]l [D]m
where Kf and Kb are proportionality constants.
At equilibrium, Rate of forward reaction (rf) = Rate of backward reaction (rb)
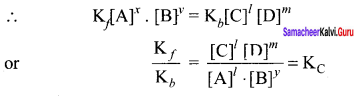
where KC is the equilibrium constant in terms of concentration. At a given temperature, the ratio of the product of active masses of reaction products raised to the respective stoichiometric coefficients in the balanced chemical equation to that of the reactants is a constant known as equilibrium constant.
If the reactants and products of the above reaction are in gas phase, then the equilibrium constant can be written in terms of partial pressures
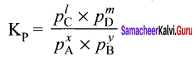
where PA, PB, PC and PD are the partial pressure of gases A, B, C and D respectively.
Question 2.
Derive the values of K and K for the synthesis of HI.
Answer:
H2(g) + I2(g) \(\rightleftharpoons\) 2HI(g)
Let us consider the formation of HI in which ‘a’ moles of hydrogen, ‘b’ moles of iodine gas are allowed to react in an container of volume ‘V’. Let ‘x’ moles of each of H2, and I2, react together to form 2x moles of HI.
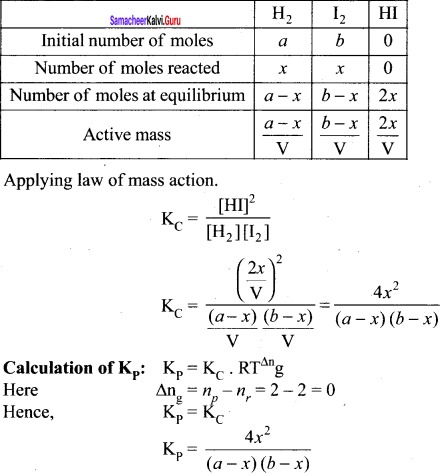
Question 3.
Derive the values of K and K for dissociation of PCI5.
Answer:
Consider that ‘a’ moles of PCl5 is taken in container of volume ‘V’ Let x moles of PCI5 be dissociated into x moles of PCI3 and x moles of Cl2.
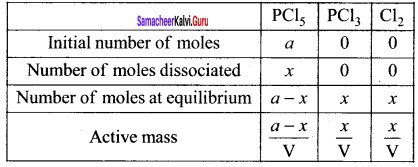
Applying law of mass action
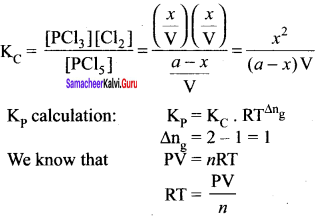
Where ‘n’ is the total number of moles at equilibrium
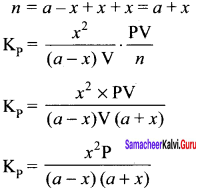
Question 4.
At certain temperature and under a pressure of 4 atm, PCl5 is 10% dissociated. Calculate the pressure at which PCI5 will be 20% dissociated at temperature remaining constant.
Calculation of Kp
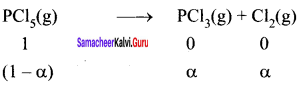
Total no. of moles in the equilibrium mixture = 1 – α + α + α = (1 + α) mol.
Let the total pressure of equilibrium mixture = ρ atm
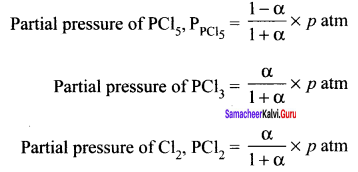
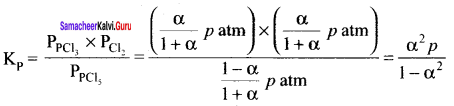
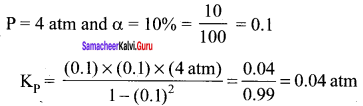
calculation of P under new condition
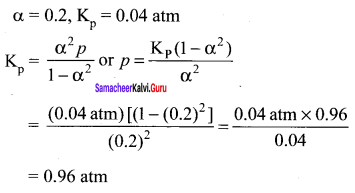
IV. Numerical Problems
Question 1.
Find the value of K for each of the following equlibria from the value of K
(a) 2NOCI(g) \(\rightleftharpoons\) 2NO(g) + Cl2(g); Kp= 1.8 x 102 atm at 500 K
(b) CaCO3(s) \(\rightleftharpoons\) CaO(s) + CO2(g): Kp = 167 atm at 1073 K.
Answer:
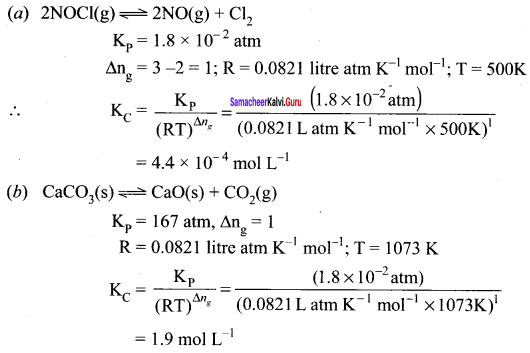
Question 2.
What is the equilibrium concentration of each of the substances in the equilibrium when the initial concentration of ICI was 0.78 M?
2ICI(g) \(\rightleftharpoons\) I2(g) + CI2(g); KC = 0.14
Answer:
Suppose at equilibrium, the molar concentration of both I2(g) and Cl2(g) is x mol L-1.
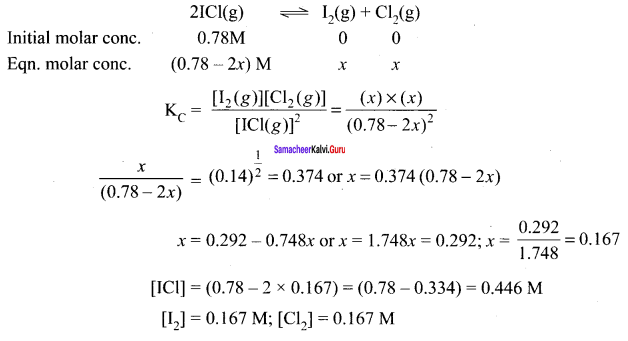
Question 3.
Equilibrium constant K for the reaction. N2(g) + 3H2(g) \(\rightleftharpoons\) 2NH3(g) at 500 K is 0.061. At particular time, the analysis shows that the composition of the reaction mixture is 3.0 mol L-1 of N2; 2.0 mol L-1 of H2; 0.50 moI L-1 of NH3. Is the reaction at equilibrium?
Answer:
The given reaction is: N2(g) + 3H2(g) \(\rightleftharpoons\) 2NH3(g)
According to available data
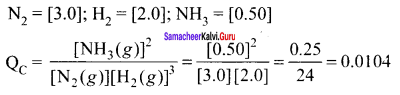
Common Errors
- In writing Kp, KC values from the equations, students may confuse to write whether products or reactants in the numerator or in the denominator
- When Kp = KC.(RT)0 students may confuse.
- In writing An values, students will consider all reactants and products.
- Students are confused to understand the concept of Q value and KC value.
- In writing chemical equilibrium reaction, you may miss the physical states of reactants and products.
- ∆ngvalue calculation will go wrong if you consider all the reactants and products.
- Equilibrium constant value is calculated under equilibrium condition and reaction quotient value defined at sometimes at equilibrium condition wrongly by the students.
- Chemical equilibrium condition must be known. Students may wrongly write in an open vessel, the equilibrium take place.
- Equilibrium symbol, students may wrongly write as =
Rectifications
- Always we have to write Kp & KC as
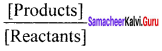
- (Anything)° = 1. So Kp = KC.
- Only they have to consider gaseous reactants and gaseous products. Since solid and liquid are constant in their concentration.
- Q value is calculated under non equilibrium conditions. KC value is calculated under equilibrium condition.
- Physical states of reactants and products must be written as a subscript by the words s, l, g. (solid, liquid, gas)
- ∆ng = ngp – ngr You should consider only gaseous products and gaseous reactants.
- Reaction quotient Q value is calculated only under non-equilibrium conditions.
- Chemical equilibrium reactions are always take place at closed vessel only.
- Equilibrium reaction symbol is ⇔.
We as a team believe the information prevailing regarding the Samacheer Kalvi Solutions for 11th Chemistry Chapter 8 Physical and Chemical Equilibrium has been helpful in clearing your doubts to the fullest. For any other help do leave us your suggestions and we will look into them. Stay in touch to get the latest updates on Tamilnadu State Board Solutions for different subjects in the blink of an eye.