Tamilnadu Samacheer Kalvi 11th Chemistry Notes Chapter 13 Hydrocarbons Notes
Alkenes – Alkenes are unsaturated hydrocarbons that contain carbon-carbon double bond. They are represented by the general formulae CnH2n where n stands for the number of carbon atoms in the molecule. Alkenes are also known as olefins.
Geometrical isomerism – It is a type of stereoisomerism and it is also called cis-trans isomerism or geometrical isomerism. Such type of isomerism results due to the restricted rotation of doubly bonded carbon atoms.
MarkovnikofFs rule – When an unsymmetrical alkene reacts with hydrogen halide, the hydrogen atom adds to the carbon that has more number of hydrogen atoms and halogen adds to the carbon atom having fewer hydrogen atoms.
Kharasch addition – Metal catalysed free radical addition of CXCl3 compounds to alkenes is called Kharasch addition.
Polymerisation – A polymer is a large molecule formed by the combination of large number of small molecules. The process is known as polymerisation.
Alkynes – Alkynes are unsaturated hydrocarbons that contain carbon-carbon triple bond in their molecules. Their general molecular formula is CnH2n-2.
Ozonolysis – Ozone adds to carbon-carbon triple bond of alkynes to form ozonides. This process is known as ozonolysis.
Aromaticity – A compound may be aromatic, if it obey the following rules,
The molecule must be co-planar.
Complete delocalisation of π electrons in the ring.
Presence of (An + 2)π electrons in the ring, where n is an integer (n = 0,1,2 …). This is known as Huckel’s rule.
Ortho and para directing groups – OH, – NH2, – NHR, – CH3 – OCH3 etc.
Meta directing groups NO2, – CN, – CHO, – COOH etc.
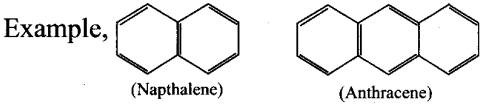
Polynuclear aromatic hydrocarbons – Two or more benzene rings fused to form polynuclear aromatic hydrocarbons.