Tamilnadu Samacheer Kalvi 11th Chemistry Notes Chapter 12 Basic Concepts of Organic Reactions Notes
Organic reactions – Substrate is an organic molecule reacts with reagent, which may be an organic, inorganic or any agent like heat, photon etc that brings about the chemical change to form a product. This is known as organic reactions.
Mechanism of the reaction – The series of simple steps which collectively represent the chemical change, from substrate to product is called as the mechanism of the reaction.
Type of fission of a covalent bonds – (i) Homolytic fission, and (ii) Heterolytic fission.
Homolytic cleavage –
- Homolytic cleavage is the process in which a covalent bond breaks symmetrically in such way that each of the bonded atoms retains one electron.
- This type of cleavage occurs under high temperature or in the presence of UV-light.
- In such molecules, the cleavage of bonds results into free radicals.
- Free radicals are short lived and highly reactive species.
Free radical initiators – The type of reagents that promote homolytic cleavage in substrate are called as free radical initiators.
Examples for free radical initiators –
- Azobisisobutyronitrile (AIBN)
- Benzoyl peroxide
Heterolytic cleavage –
- Heterolytic cleavage is the process in which a covalent bond breaks unsymmetrically such that one of the bonded atoms retains the bonded pair of electrons.
- It results in the formation of a cation and an anion.
Carbocation – In a carbocation, the carbon atom bearing positive charge. It is sp2 hybridised and hence it has a planar structure.
Carbanion – In a carbanion,, the carbon atom bearing negative charge. It is sp3 hybridised and hence it is pyramidal in shape.
Nucleophiles – Nucleophiles are reagents that has high affinity for electropositive centers. They possess an atom has an unshared pair of electrons. They are usually negatively charged ions or electron rich neutral molecules.
Nucleophilic reagents – Ammonia, amines, water, alcohols, ethers, hydrogen sulphide, thiols.
Electrophiles – Electrophiles are reagents that are attracted towards negative charge or electron rich center. They are either positively charged ions or electron deficient neutral molecules.
Electrophilic reagents – Carbon dioxide, Aluminium chloride, borontrifluoriuc and ferric chloride.
Electron movement in organic reactions – There are three types of electron movement,
- Lone pair becomes a bonding pair.
- Bonding pair becomes a lone pair.
- a bond breaks and becomes another bond.
Electron displacement effects in covalent bonds – Electron displacement effects in covalent bonds occurs due to the presence of an atom or group of different electronegativity or under the influence of some outside attaching group. The electron displacements are categorized into, Inductive effects, Resonance effect, electromeric effect and hyper conjugation.
Inductive effect (I) – It is defined as the change in the polarization of a covalent bond due to the presence of adjacent bonded atoms or groups in the molecule.
+1 effect – Atoms or groups which lose electron towards a carbon atoms are said to have a +1 effect. Example, (CH3)3C-, (CH3)2CH-, CH3-CH2-, CH3
-I effect – Atoms or groups which draw electrons away from a carbon atom one said to have a -I effect. Example, -NO2, F–, Cl–, Br–, I– , -OH, C6H5–
Electromeric effect – The electromeric effect refers to the polarity produced in a multiple bonded compound when it is attacked by a reagent when a double or a triple bond is exposed to an attack by an electrophile the two n electrons which from the 7t bond are completely transferred to one atom or the other.
Resonance or mesomeric effect – Certain organic compounds can be represented by more than one structure and they differ only in the position of bonding and lone pair of electrons. Such structure are called resonance (or) canonical structure. This phenomenon is also called resonance effect.
Positive resonance effect – Those atoms or groups which lose electrons towards a carbon atom are said to have a + M or + R effect.
Example, -Cl, -Br, -I, -NH2, -NR2, -OH, -OCH2
Negative resonance effect – Those atoms or groups which draw electrons away from a carbon atom are said to have a -M or -R effect.
Example:
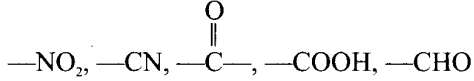
Hyper conjugation – The delocalization of electron of a-bond is called as hyper conjugation.
Substitution reaction – In this reaction an atom or a group of atoms attached to a carbon atom is replaced by a new atom or a groups of atoms.
Addition reaction – It is a characteristic reaction of an unsaturated compound. In this reaction two molecules combine to give a single product.
Elimination reaction – In this reaction two substituents are eliminated from the molecule, and a new C-C double bond is formed between the carbon atoms to which the eliminated atoms\groups are previously attached.
Oxidation and reduction reactions – Most of the oxidation reaction of organic compounds involves gain of oxygen or loss of hydrogen. Reduction involves gain of hydrogen and loss of oxygen.
Functional group inter conversion – Organic synthesis involves functional group inter conversions. A particular functional group can be converted into other functional group by reacting it with suitable reagents.