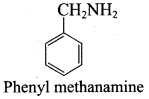
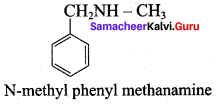
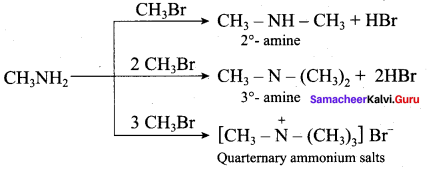
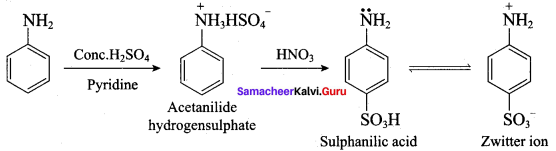
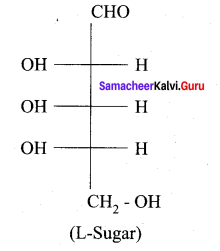
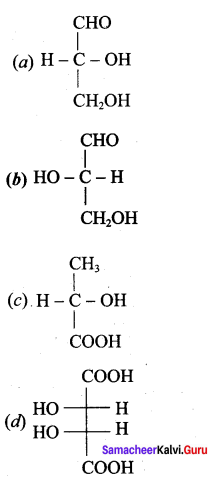
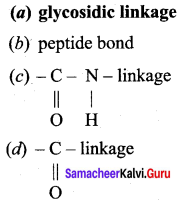
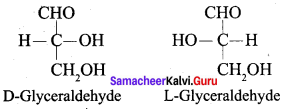
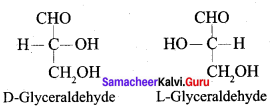
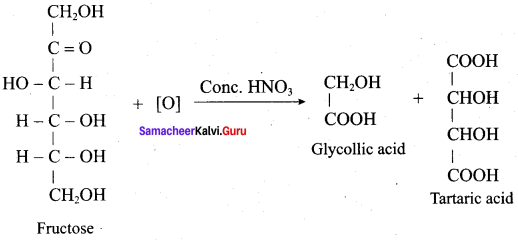
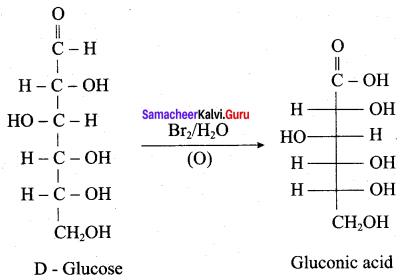
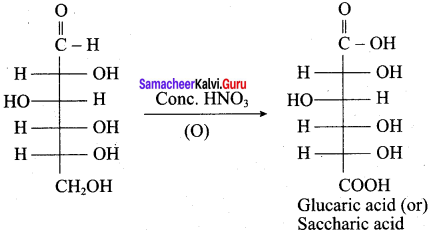
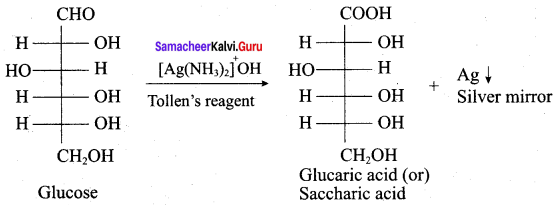
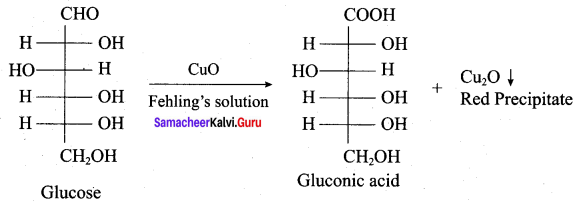
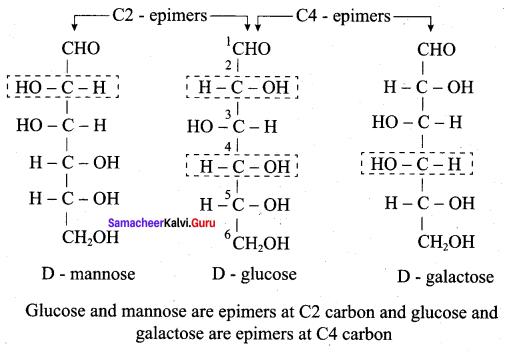
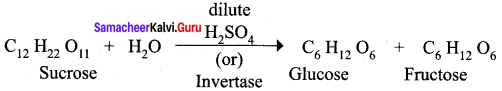
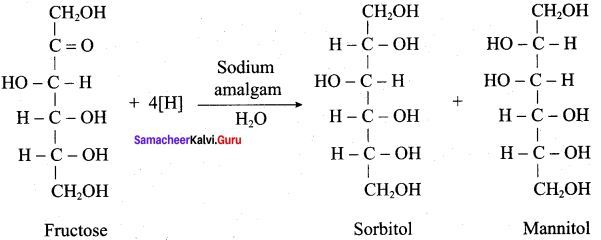
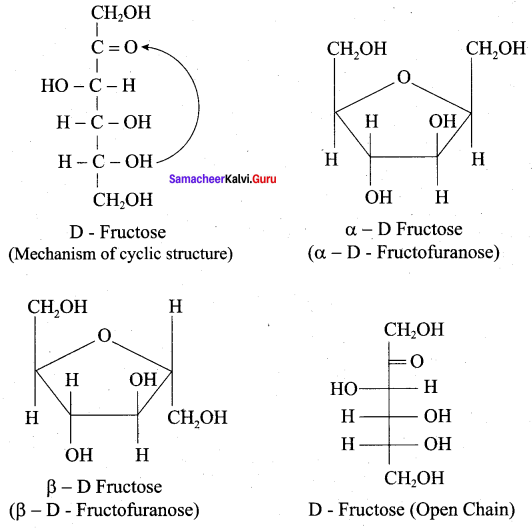
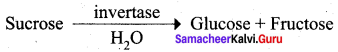
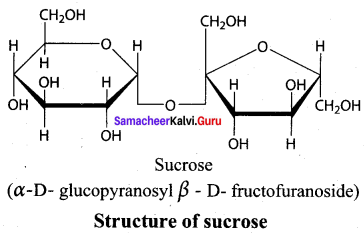
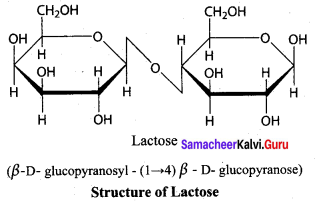
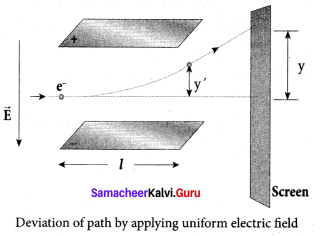
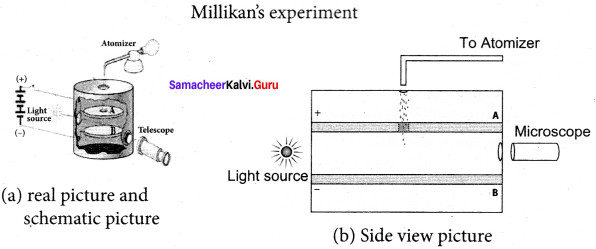
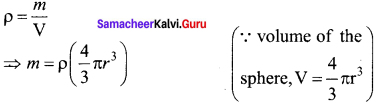
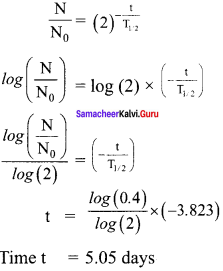
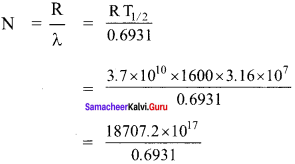
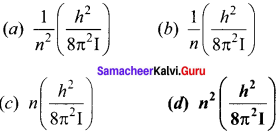
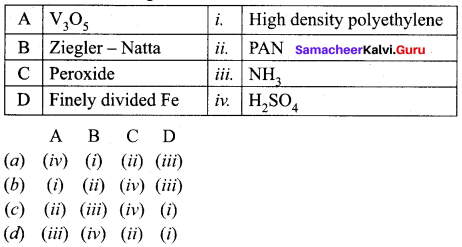
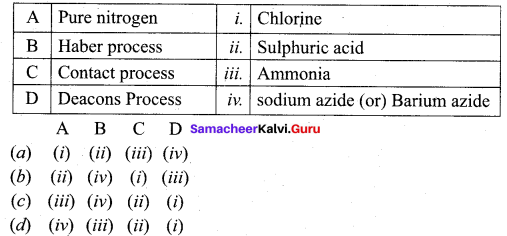
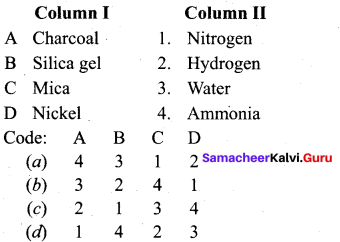
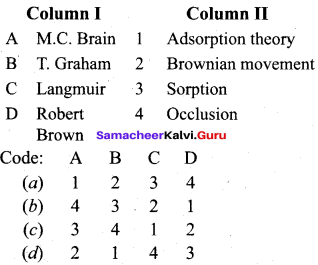
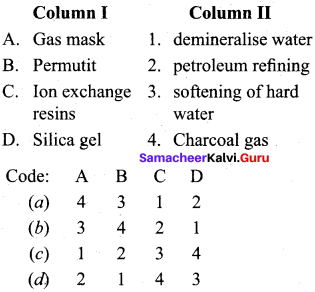
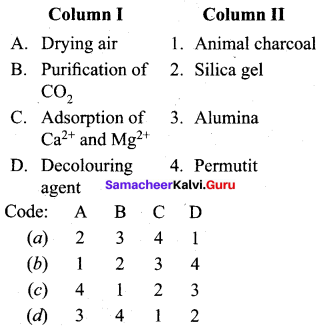
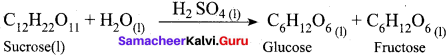
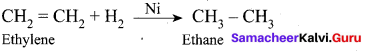
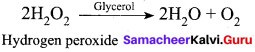
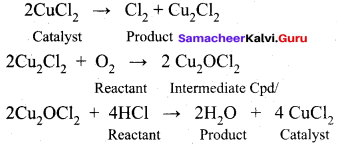
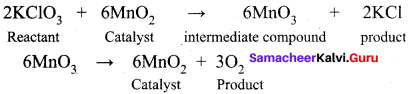
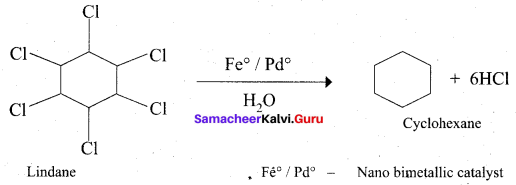
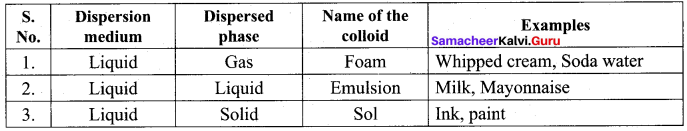
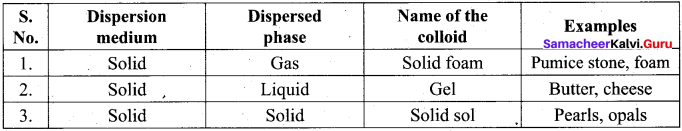
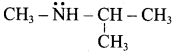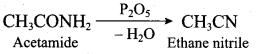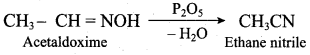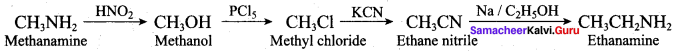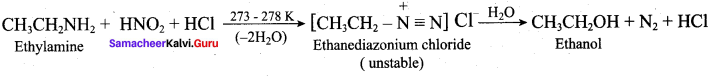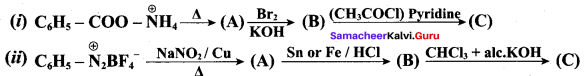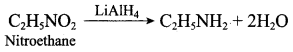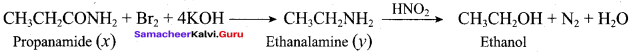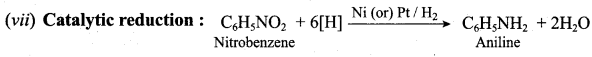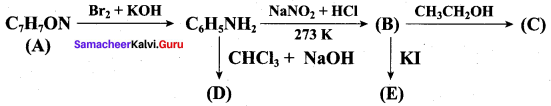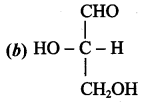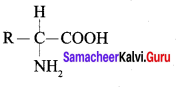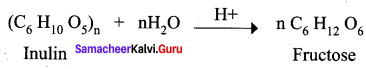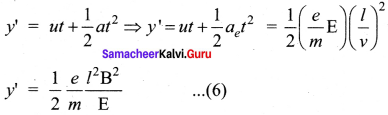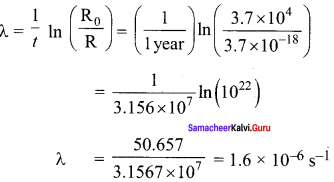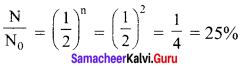Get Tamilnadu Board Class 12 Bio Zoology Solutions Chapter wise Study Material to score good marks in the exam. Various chapters with subtopics are explained clearly in Samacheer Kalvi Class 12 Bio Zoology Solutions Material. All the Samacheer Kalvi Class 12 Bio Zoology Book Solutions Chapter 1 Reproduction in Organisms Questions, answers, Notes, Guide, Pdf along with the explanations are provided by the subject experts. Students can easily learn Tamilnadu Board Class 12 Chapter wise Bio Zoology with the help of the step by step guide provided on our site. Learn all the Samacheer Kalvi Class 12 Bio Zoology concepts to attempt the exam with more confidence. Read all the concepts of Tamilnadu Board Solutions for Class 12 Bio Zoology Chapter 1 Reproduction in Organisms.
Tamilnadu Samacheer Kalvi 12th Bio Zoology Solutions Chapter 1 Reproduction in Organisms
Whether you want to become an expert in Bio Zoology or to get good marks in the exam, you have one and only solution is practicing with Samacheer Kalvi 12th Bio Zoology Chapter wise Solutions. Strengthen your weakness by learning the Samacheer Kalvi 12th Bio Zoology Chapter 1 Reproduction in Organisms Questions and Answers on our site. You can learn directly online on our website or learn offline by downloading Samacheer Kalvi 12th Bio Zoology Chapter wise material. Save your time and read Samacheer Kalvi 12th Bio Zoology Subject at your comfort level.
Samacheer Kalvi 12th Bio Zoology Reproduction in Organisms Text Book Back Questions and Answers
Question 1.
In which type of parthenogenesis are only males produced?
(a) Arrhenotoky
(b) Thelytoky
(c) Amphitoky
(d) Both a and b
Answer:
(a) Arrhenotoky
Question 2.
Animals giving birth to young ones:
(a) Oviparous
(b) Ovoviviparous
(c) Viviparous
(d) Both a and b
Answer:
(c) Viviparous
Question 3.
The mode of reproduction in bacteria is by ___________
(a) Formation of gametes
(b) Endospore formation
(c) Conjugation
(d) Zoospore formation
Answer:
(c) Conjugation
Question 4.
In which mode of reproduction variations are seen?
(a) Asexual
(b) Parthenogenesis
(c) Sexual
(d) Both a and b
Answer:
(c) Sexual
Question 5.
Assertion and reasoning questions:
In each of the following questions there are two statements. One is assertion (A) and other is reasoning (R). Mark the correct answer as
(a) If both A and R are true and R is correct explanation for A
(b) If both A and R are true but R is not the correct explanation for A
(c) If A is true but R is false
(d) If both A and R are false
I. Assertion: In bee society, all the members are diploid except drones.
Reason: Drones are produced by parthenogenesis.
A B C D
II. Assertion: Off springs produced by asexual reproduction are genetically identical to the parent.
Reason: Asexual reproduction involves only mitosis and no meiosis.
A B C D
III. Assertion: Viviparous animals give better protection to their off springs.
Reason: They lay their eggs in the safe places of the environment.
A B C D
Answer:
(I) A (II) A (III) C
Question 6.
Name an organism where cell division is itself a mode of reproduction.
Answer:
Amoeba
Question 7.
Name the phenomenon where the female gamete directly develops into a new organism with an avian example.
Answer:
Parthenogenesis is the phenomenon where the unfertilized female gamete (egg) develops into a new individual,
e.g. Turkey
Question 8.
What is parthenogenesis? Give two examples from animals.
Answer:
Development of an egg into a complete individual without fertilization is known as parthenogenesis. It was first discovered by Charles Bonnet in 1745.
E.g. Honey bees, Aphis.
Question 9.
Which type of reproduction is effective – Asexual or sexual and why?
Answer:
Sexual reproduction is highly effective than asexual reproduction since the off springs produced are genetically different from parents causing variations. Variation leads to evolution.
Question 10.
The unicellular organisms which reproduce by binary fission are considered immortal. Justify.
Answer:
In unicellular organisms during binary fission, the entire cell (organism) divides completely to form two daughter cells which later on develop into adult and the process goes on repeatedly during each division leading to immortality of cell (organism). Hence unicellular organisms like amoeba are ‘biologically immortal’.
Question 11.
Why is the offspring formed by asexual reproduction referred as a clone?
Answer:
Off springs developed by asexual reproduction are referred as clones since they are genetically & morphologically similar to this parent.
Question 12.
Why are the off springs of oviparous animal at a greater risk as compared to off springs of viviparous organisms?
Answer:
Oviparous animals are egg-layers. The eggs containing embryo are laid out of their body and are highly susceptible to environmental factors (temperature, moisture etc.) and predators. Whereas, in viviparous animals, the embryo develops inside the body of female and comes out as young ones. Hence off springs of oviparous animals are at risk compared to viviparous animal.
Question 13.
Give reasons for the following:
- Some organisms like honey bees are called parthenogenetic animals
- A male honey bee has 16 chromosomes whereas its female has 32 chromosomes
Answer:
- Among honey bees, the queen bee and worker bees develop from fertilized eggs whereas the drones develop from unfertilized eggs. Hence the honey bees are parthenogenetic animals showing incomplete parthenogenesis.
- Female honey bees (Queen or worker bees) are diploid having 32 chromosomes since they develop from the fertilized egg. On the other hand, the male honey bees (drones) develop from unfertilized egg possessing only 16 chromosomes (i.e., Haploid)
Question 14.
Differentiate between the following:
(a) Binary fission in amoeba and multiple fission in Plasmodium
(b) Budding in yeast and budding in Hydra
(c) Regeneration in lizard and Planaria
Answer:
(a) Binary fission in amoeba and multiple fission in Plasmodium
- Binary fission in amoeba: In binary fission of amoeba, the plane of division is hard to observe. The nucleoli disintegrates. The nucleus divides mitotically forming two nucleus. The cell constricts in middle, so the cytoplasm divides forming two daughter cells.
- Multiple fission: In Multiple fission of plasmodium, the oocyte or schizont divides into many similar daughter cells simultaneously. Nucleus undergoes repeated mitosis producing many nuclei without the division of cytoplasm. Later the cytoplasm divides & encircles each nucleus forming many daughter cells oocyte undergoes sporogony forming sporozoites. Schizont undergoes schizogony forming merozoites.
(b) Budding in yeast and budding in Hydra:
- Budding in Yeast: Yeast is xxxx cellular organism in which the bud develops as a small protuberance following the nuclear division and finally detached to new individual.
- Budding in Hydra: Hydra is a multicellular organism where the bud xxxxxx from the parents body, grows gradually and finally gets detached.
(c) Regeneration in lizard and Planaria:
- Regeneration of Lizard: If the tail of the lizard is cut and removed, a new tail will regenerate in damaged part. In lizard only the new tail is regenerated.
- Regeneration of Planaria: If a planarian worm get cut then each half regenerates the lost part resulting in two worms.
In planaria, the cut removed part developed into an entire worm.
Question 15.
How is juvenile phase different from reproductive phase?
Answer:
- Juvenile phase: Juvenile phase is the period of growth between the brith of an organism and before its reproductive maturity.
- Reproduction phase: Reproductive phase is the period of growth after juvenile phase when an individual attain reproductive maturity and reproduces.
Question 16.
What is the difference between syngamy and fertilization?
Answer:
Syngamy & fertilization both are more similar terms with a difference that syngamy refers to the process of fusion of two gametes forming zygote while fertilization refers to the process of being fertile.
Samacheer Kalvi 12th Bio Zoology Reproduction in Organisms Additional Questions and Answers
1 – Mark Questions
I. Choose the Correct Answer
Question 1.
Transverse binary fission is noticed in ___________
(a) Amoeba
(b) Planaria
(c) Ceratium
(d) Vorticella
Answer:
(b) Planaria
Question 2.
Multiple fission occurring in the oocyte of plasmodium is called ___________
(a) Schizogony
(b) Merogamy
(c) Syngamy
(d) Sporogony
Answer:
(d) Sporogony
Question 3.
Taenia solium requires ___________ as a secondary host to complete its life cycle.
(a) Mosquito
(b) pig
(c) dog
(d) human
Answer:
(b) pig
Question 4.
Which type of parthenogenesis only females are produced?
(a) Arrhenotoky
(b) Amphitoky
(c) Thelytoky
(d) Both (a) and (b)
Answer:
(c) Thelytoky
Question 5.
Which among the following animal is not a continuous breeder?
(a) Hen
(b) Rabbit
(c) Honey bees
(d) Frogs
Answer:
(d) Frogs
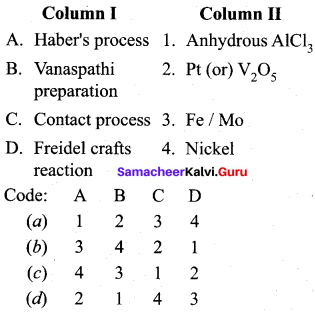
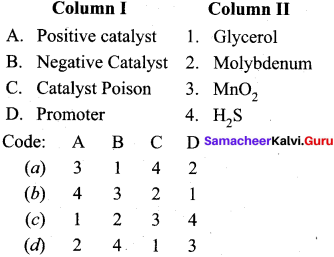
Question 6.
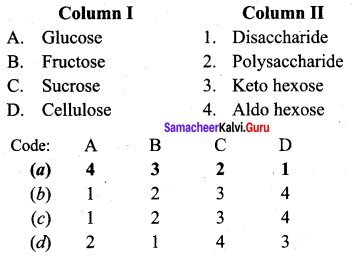
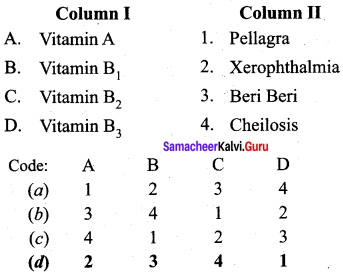
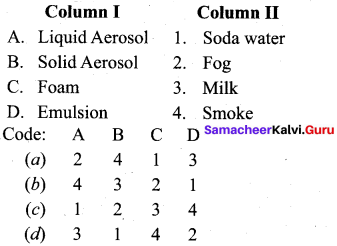
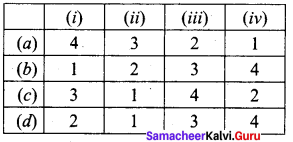
Match the following.

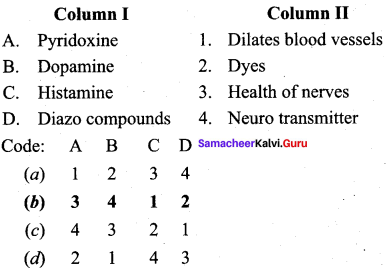
Answer:
a – iii, b – iv, c – ii, d – i
Question 7.
Identify the incorrect statement regarding parthenogenesis.
(a) Development of sperm without fertilization.
(b) It was first discovered by Charles Bonnet.
(c) Honey bees exhibit incomplete parthenogenesis.
(d) Amphitoky is a type of natural parthenogenesis.
Answer:
(a) Development of sperm without fertilization.
Question 8.
Oblique binary fission is seen in ___________
Answer:
Dinoflagellates
Question 9.
The process by which gravid proglottids of tapeworm gets cut off is called ___________
Answer:
apolysis
Question 10.
The concept of regeneration was first noticed in ___________
Answer:
Hydra
Question 11.
Fusion of small sized, morphologically different gametes is called ___________
Answer:
merogamy
Question 12.
Identify the wrong statement.
(a) Oviparous animals lays eggs.
(b) Viviparous animals give rise to young ones.
(c) Ovoviviparous animals lays eggs and then hatch it to young ones.
(d) Amphibians are oviparous animals.
Answer:
(c) Ovoviviparous animals lays eggs and then hatch it to young ones.
Question 13.
Assertion (A): Organisms show three phases in their life cycle.
Reason (R): Juvenile phase is a degenerative phase.
(a) A is correct R but is incorrect.
(b) Both A and R are correct
(c) R is the correct explanation for A
(d) A is not correct but R is correct
Answer:
(a) A is correct R but is incorrect.
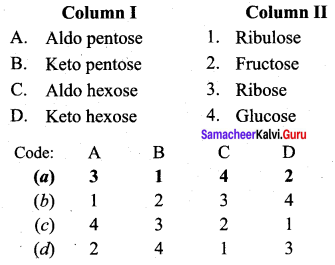
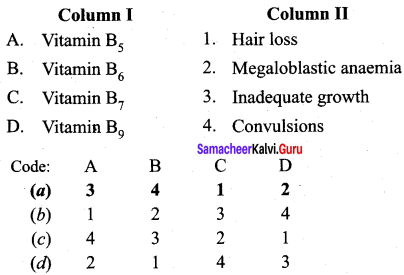
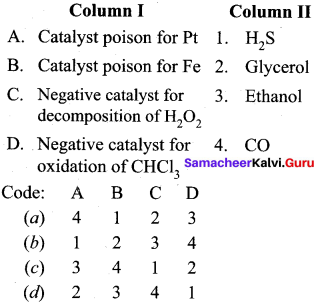
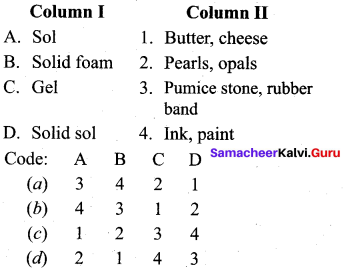
Question 14
Match the statements.

Answer:
a – iii, b – iv, c-i, d- ii.
Question 15.
The ploidy of males produced by arrhenotoky parthenogenesis is
Answer:
haploid
Question 16.
Identify the mismatched pair.
(a) Paedogenesis – Liver fluke
(b) Strobilation – Aurelia
(c) Amphitoky – Honey bee
(d) Encystment – Amoeba
Answer:
(c) Amphitoky – Honey bee
Question 17.
Identify the proper sequence.
(a) juvenile phase, senescent phase, vegetative phase
(b) juvenile phase, maturity phase, senescent phase
(c) vegetative phase, maturity phase, juvenile phase
(d) senescent phase, juvenile phase, vegetative phase
Answer:
(b) juvenile phase, maturity phase, senescent phase
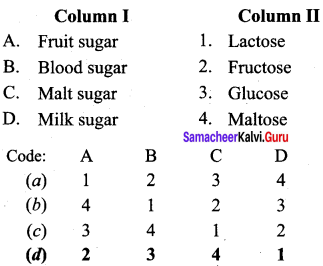
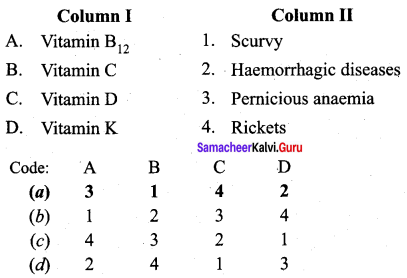
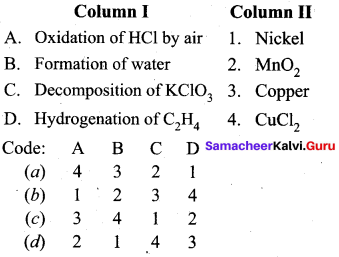
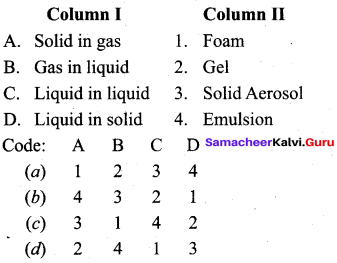
Question 18.
Match the following:

Answer:
a – iv, b – iii, c – ii, d – i
Question 19.
Which of the following types of asexual reproduction is noticed in Amoeba?
(a) Sporulation
(b) Encystment
(c) Binary fission
(d) All the above
Answer:
(d) All the above
Question 20.
Pick out the organism whose fertilization occurs internally.
(a) reptiles
(b) sponges
(c) pisces
(d) amphibians
Answer:
(a) reptiles
Question 21.
Assertion (A): Asexual reproduction is called blastogenic reproduction.
Reason (R): It is accomplished by mitotic and meiotic divisions.
(a) A and R are correct
(b) A is correct but R is incorrect
(c) Both A and R are incorrect
(d) R is the correct explanation for A
Answer:
(6) A is correct but R is incorrect
Question 22.
Egg laying hen is an example for ___________
(a) Thelytoky
(b) Ovovivipary
(c) Vivipary
(d) Ovipary
Answer:
(d) Ovipary
Question 23.
Assertion (A): Syngamy refers to the fusion of two haploid gametes.
Reason (R): Syngamy leads to zygote formation.
(a) A and R are correct.
(b) A and R are incorrect.
(c) R is not the right explanation for A
(d) A is correct but R is incorrect.
Answer:
(a) A and R are correct.
Question 24.
Human beings are an example for ___________ breeders.
Answer:
continuous
2 – Mark Questions
Question 1.
Why asexual reproduction is called as somatogenic reproduction?
Answer:
Asexual reproduction is usually by amitotic or mitotic division of the somatic (body) cells, – hence is also known as somatogenic or blastogenic reproduction.
Question 2.
Name the four types of fission seen in animals.
Answer:
Binary fission, Multiple fission, Sporulation and Strobilation.
Question 3.
Define fission.
Answer:
Fission is the division of the parent body into two or more identical daughter individuals.
Question 4.
Differentiate between transverse binary fission and longitudinal binary fission.
Answer:
Transverse binary fission:
- Plane of division runs along the transverse axis of the organism
- Example: Paramecium
Longitudinal binary fission:
- Plane of division runs along the longitudinal axis of the organism.
- Example: Euglena
Question 5.
Define Plasmotomy with example.
Answer:
Plasmotomy is the division of multinucleated parent into many multinucleate daughter individuals with the division of nuclei. Nuclear division occurs later to maintain normal number of nuclei. Plasmotomy occurs in Opalina and Pelomyxa (Giant Amoeba).
Question 6.
What do you mean by regeneration in living organisms? Mention its types.
Answer:
Regeneration is the regrowth of injured region.
It is of two types:
- Morphallaxis
- Epimorphosis.
Question 7.
How is the fertilization of amphibians differs from aves based on site?
Answer:
In amphibians the fertilization is external (taking place outside the body of female organism) whereas internal fertilization takes place in aves.
Question 8.
What is Paedogamy?
Answer:
Paedogamy is the sexual union of young individuals produced immediately after the division of the adult parent cell by mitosis.
Question 9.
Write a brief note on conjugation.
Answer:
Conjugation is the temporary union of the two individuals of the same species. During their union both individuals, called the conjugants exchange certain amount of nuclear material (DNA) and then get separated. Conjugation is common among ciliates,
e.g. Paramecium, Vorticella and bacteria (Prokaryotes).
Question 10.
Classify animal breeding based on time.
Answer:
On the basis of time, breeding animals are of two types: seasonal breeders and continuous breeders. Seasonal breeders reproduce at particular period of the year such as frogs, lizards, most birds, deers etc., Continuous breeders continue to breed throughout their sexual maturity
e.g. honey bees, poultry, rabbit etc.
Question 11.
Define Vivipary.
Answer:
Vivipary is a condition in which animals give rise to live young ones after being nourished in the uterus though the placenta.
E.g. human.
Question 12.
List out the four types of binary fission.
Answer:
- Simple irregular binary fission
- Transverse binary fission
- Longitudinal binary fission
- Oblique binary fission
Question 13.
Repeated fission is a type of multiple fission. Yes or No? Why?
Answer:
Yes. If multiple fission produces four or many daughter individuals by equal cell division and the young ones do not separate until the process is complete, then this division is called repeated fission
e.g. Vorticella.
Question 14.
Define apolysis.
Answer:
The detachment of gravid proglottids either singly or in groups from the body of tapeworm is called apolysis.
3 – Mark Questions
Question 15.
Compare schizogony with sporogony of plasmodium.
Answer:
Schizogony:
- In schizogony, the multiple fission occurs in the schizont.
- It results in the formation of merozoites.
Sporogony:
- In sporogony, the multiple fission occurs in the oocyte.
- It results in the formation of sporozoites.
Question 16.
Write a short note on encystment in amoeba.
Answer:
During unfavorable conditions (increase or decrease in temperature, scarcity of food) Amoeba withdraws its pseudopodia and secretes a three-layered, protective, chitinous cyst wall around it and becomes inactive. This phenomenon is called encystment. When conditions become favourable, the encysted Amoeba divides by multiple fission and produces many minute amoebae called pseudopodiospore or amoebulae.
The cyst wall absorbs water and breaks off liberating the young pseudopodiospores, each with a fine pseudopodia. They feed and grow rapidly to lead an independent life.
Question 17.
How exogenous buds are developed by Hydra?
Answer:
When buds are formed on the outer surface of the parent body, it is known as exogenous budding e.g. Hydra. In Hydra when food is plenty, the ectoderm cells increase and form a small elevation on the body surface. Ectoderm and endoderm are pushed out to form the bud. The bud contains an interior lumen in continuation with parent’s gastrovascular cavity. The bud enlarges, develops a mouth and a circle of tentacles at its free end. When fully grown, the bud constricts at the base and finally separates from the parent body and leads an independent life.
Question 18.
Apolysis favours Taenia solium. How?
Answer:
In the tapeworm, Taenia solium the gravid (ripe) proglottids are the oldest at the posterior end of the strobila. The gravid proglottids are regularly cut off either singly or in groups from the posterior end by a process called apolysis. This is very significant since it helps in transferring the developed embryos from the primary host (man) to find a secondary host (pig).
Question 19.
What is autogamy?
Answer:
In autogamy, the male and female gametes are produced by the same cell or same organism and both the gametes fuse together to form a zygote
e.g. Actinosphaerium and Paramecium.
Question 20.
What is exogamy?
Answer:
In exogamy, the male and female gametes are produced by different parents and they fuse to form a zygote. So it is biparental. e.g. Human – dioecious or unisexual animal.
Question 21.
Give the definition for
- Arrhenotoky
- Thelytoky
- Amphitoky
Answer:
- Arrhenotoky: In this type only males are produced by parthenogenesis
eg: honey bees - Thelytoky: In this type of parthenogenesis only females are produced by parthenogenesis.
e.g: Solenobia - Amphitoky: In this type parthenogenetic egg may develop into individuals of any sex.
e.g: Aphis.
Question 22.
What is Incomplete parthenogenesis? Explain with example.
Answer:
Incomplete parthenogenesis is a type of reproduction in which both sexual reproduction and parthenogenesis occurs. Example: In honey bees, the fertilized eggs develop into queen bee and worker bees, whereas the unfertilized eggs develop into drones (male).
Question 23.
Explain briefly on the nature of Ovovivipary.
In Ovoviviparous animals, the embryo develops inside the egg and remains in the mother’s body until they are ready to hatch. This method of reproduction is similar to viviparity but the embryos have no placental connection with the mother and receive their nourishment from the egg yolk. Ovoviviparity is seen in fishes like shark.
Question 24.
Point out any six modes of asexual reproduction seen in animals.
Answer:
- fission
- budding
- fragmentation
- sporulation
- regeneration
- gemmule formation.
Question 25.
Enumerate the types of syngamy.
Answer:
- Autogamy
- Exogamy
- Hologamy
- Paedogamy
- Merogamy
- Isogamy
- Anisogamy
- Conjugation
Question 26.
Name the types of animals based on embryonic development with an example for each.
Answer:
- Oviparous animals
e.g. Birds - Viviparous animals
e.g. Human beings - Ovoviviparous animals
e.g. Shark
Question 27.
Write a short note on phases of life cycle.
Answer:
- Juvenile phase – Period of growth between birth of an individual and reproductive maturity.
- Reproductive phase – Period of growth when an organism attain reproductive maturity and produces new off springs.
- Senescent plane – Period of growth when the structure and functioning of body starts degenerating.
Question 28.
What is a Paedogenesis?
Answer:
In paedogenetic parthenogenesis (paedogenesis) the larvae produce a new generation of larvae ” by parthenogenesis. It occurs in the sporocysts and Redia larvae of liver fluke. It is also seen in the larvae of some insects,
e.g. Gall fly.
Question 29.
Draw and label a gemmule of sponge.
Answer:
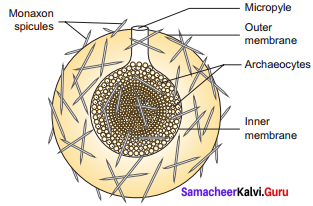
Question 30.
Differentiate between asexual and sexual reproduction.
Answer:
Asexual Reproduction:
- Involves only one parent
- Only mitotic cell division takes place.
- Off springs are genetically identical to parent.
- Gametes are not produced
Sexual Reproduction:
- Involves two parents (male & female)
- Both mitosis & meiosis takes place.
- Off springs genetically differ from the parents.
- Gametes are produced.
5 – mark Questions
Question 31.
Describe the regeneration process noticed in living organism.
Answer:
Regeneration is regrowth in the injured region. Regeneration was first studied in Hydra by Abraham Trembley in 1740. Regeneration is of two types, morphallaxis and epimorphosis. In morphallaxis the whole body grows from a small fragment e.g. Hydra and Planaria. When Hydra is accidentally cut into several pieces, each piece can regenerate the lost parts and develop into a whole new individual. The parts usually retain their original polarity, with oral ends, by developing tentacles and aboral ends, by producing basal discs.
Epimorphosis is the replacement of lost body parts. It is of two types, namely reparative and restorative regeneration. In reparative regeneration, only certain damaged tissue can be regenerated, whereas in restorative regeneration severed body parts can develop
e.g. star fish, tail of wall lizard.
Question 32.
Given an account on following terms.
- Hologamy
- Isogamy
- Anisogamy
- Merogamy
- Paedogamy
Answer:
- Hologamy: In Hologamy, the adult individuals do not produce gametes, but they themselves act as gametes and fuse to form new individuals.
E.g : Trichonympha - Isogamy : Fusion of morphologically & physiologically similar gametes.
E.g : Monocystis. - Anisogamy : Fusion of morphologically & physiologically dissimilar gametes.
Eg: Vertebrates. - Merogamy : Fusion of small sized morphologically different gametes (merogametes)
- Paedogamy : Fusion of young individuals produced immediately after the mitotic division of adult parent cell.
Higher Order Thinking Skills (HOTs) Question
Question 1.
Under threat or attack, garden lizard loses a part of its tail which trembles and avert the attention of predators, so that the lizard escapes later the tail regrown for lizard. The same phenomenon can also be noticed in organisms like starfish etc. What do you call this phenomenon? Define it.
Answer:
Regeneration is the regrowth in the injured region.
Question 2.
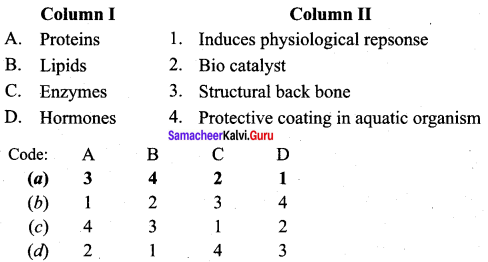
Complete the table.

Answer:
A – Only males
B – Only Females
C – Both Males & Females
Question 3.
In Vivipary, how the developing embryoes are nourished?
Answer:
In Vivipary, the embryo develops inside the womb of females body, hence they are nourished by the mother through placenta.
Question 4.
How Charles Bonnet and Abraham Trembley contributed to Biological filed?
Answer:
Charles Bonnet discovered the process of parthenogenesis. Abraham Trembley was the first to study the concept of Regeneration in the Hydra
Question 5.
‘A’ and ‘B’ are the male & female sex cells respectively which look alike and performs similar functions. ‘A’ and ‘B’ fuse to form a new individual ‘D’ Which type of gametic fusion does this represent? Give an example.
Answer:
Isogamy Eg: Monocystis
Question 6.
Complete the flow chart by mentioning the ploidy of cells in boxes.
Answer:
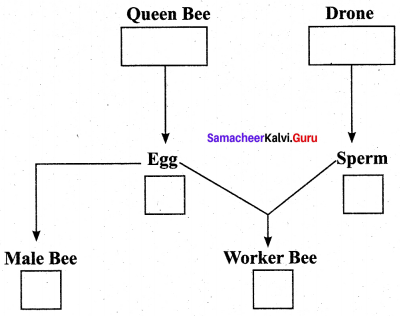
- Queen Bee Worker Bee – Diploid (2n)
- Drones (Male Bee), Egg, sperm – Haploid (n)
Question 7.
Meiosis is a type of cell division where the chromosomal number is reduced to half the number daughter cells. Which type of cellular division occurs in the drones to produces spermatozoa? Why?
Answer:
The gonadal cells of drones undergo mitosis to form sperms. Because the drones are haploid in nature since they develop from unfertilized eggs. To avoid further reduction in chromosome no. and maintain the chromosomal constancy, (instead of undergoing meiosis), mitosis will take place leading to formation of haploid gametes.
Samacheer Kalvi 12th Bio Zoology Chapter Wise Solutions PDF will help you to clear all doubts. Learn perfectly by using Samacheer Kalvi 12th Chapter 1 Reproduction in Organisms Questions and Answers Bio Zoology Solutions PDF. Leave us a comment to clear your queries. Get instant updates by bookmarking our site. Get the best learning with the effective and excellent step by step Samacheer Kalvi 12th Bio Zoology Guide.
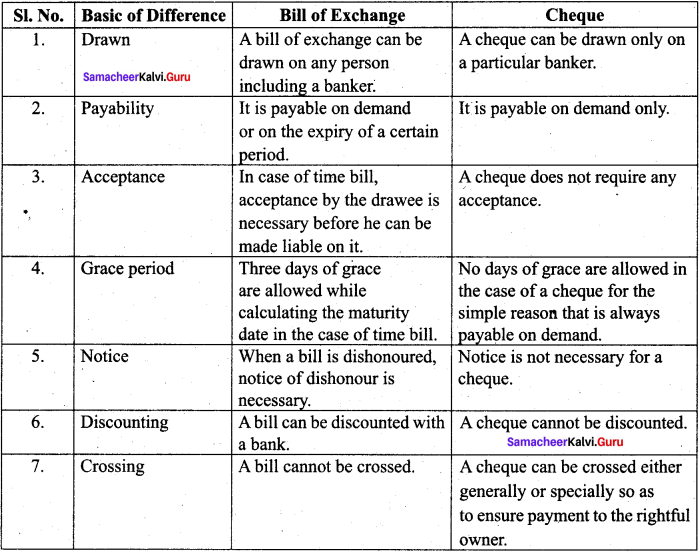
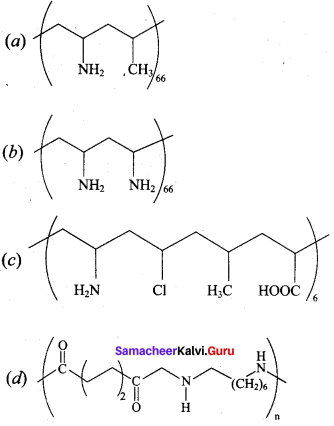
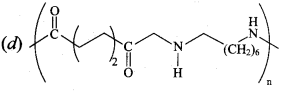
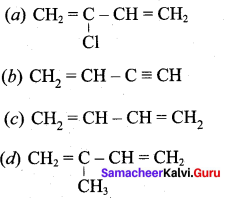
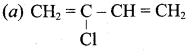
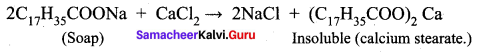
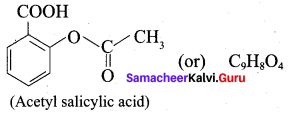
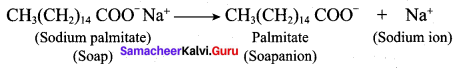
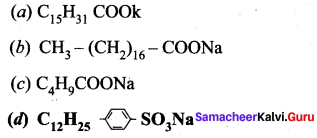
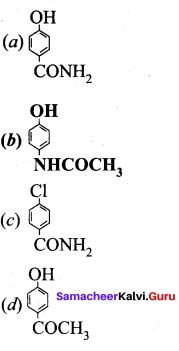
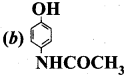
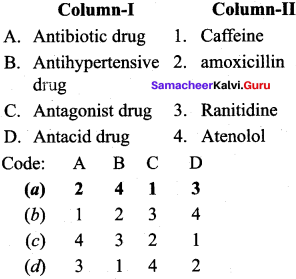
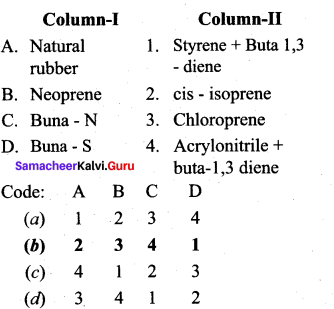
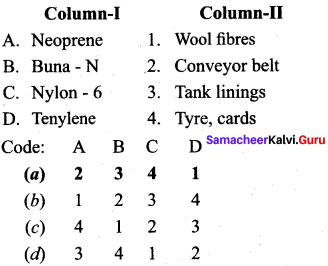
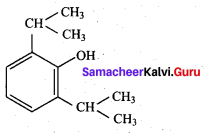
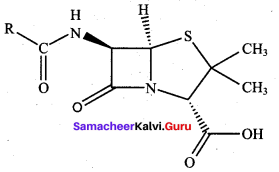
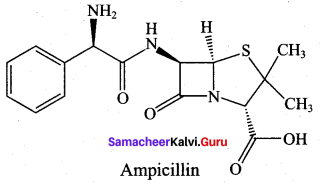
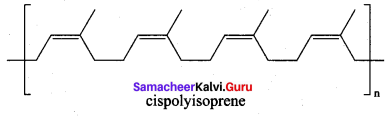
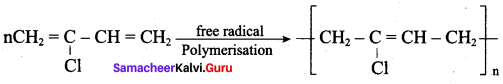

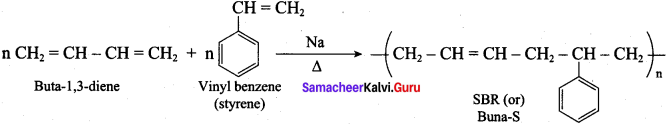
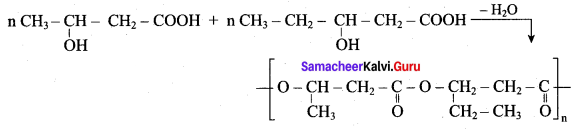

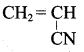
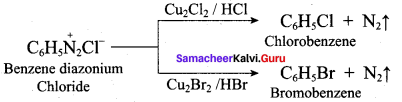
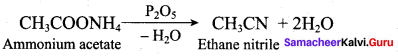
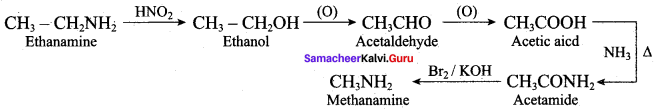
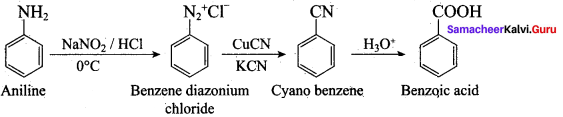
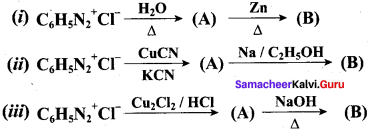
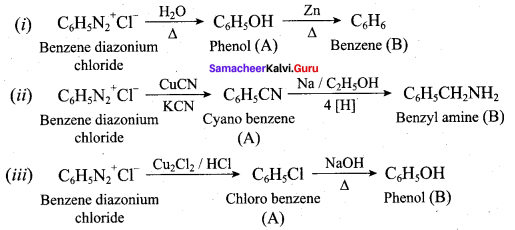
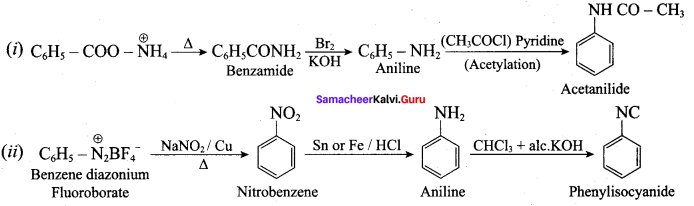
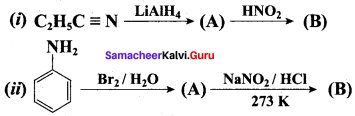
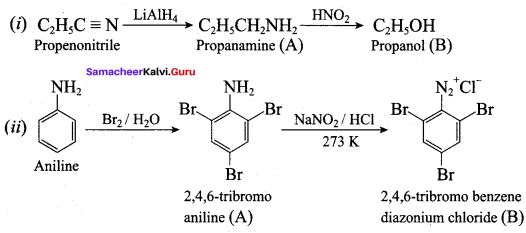
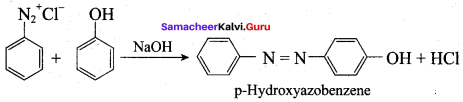
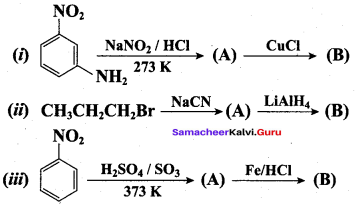
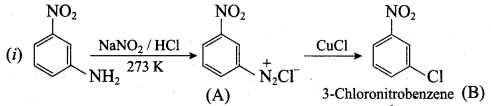
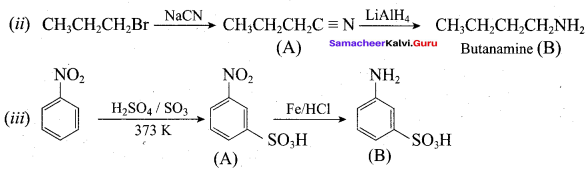
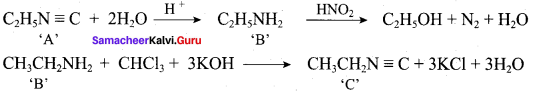
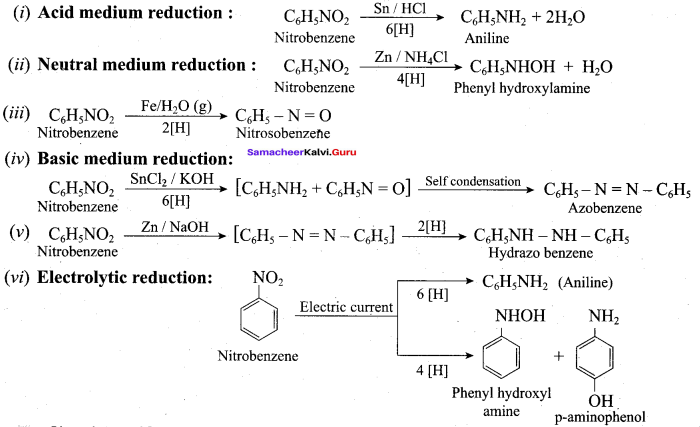
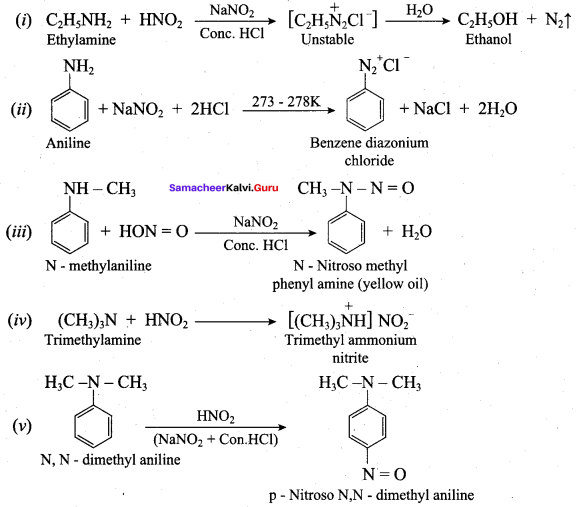
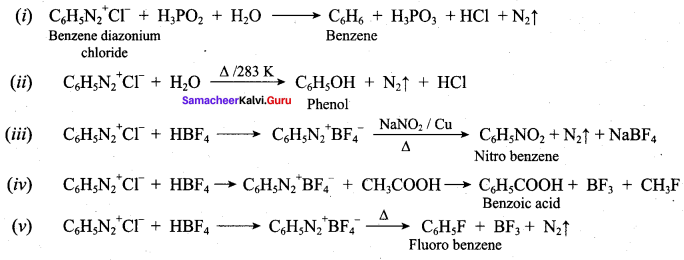
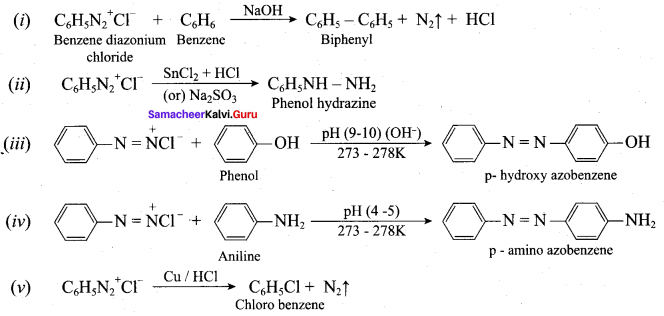
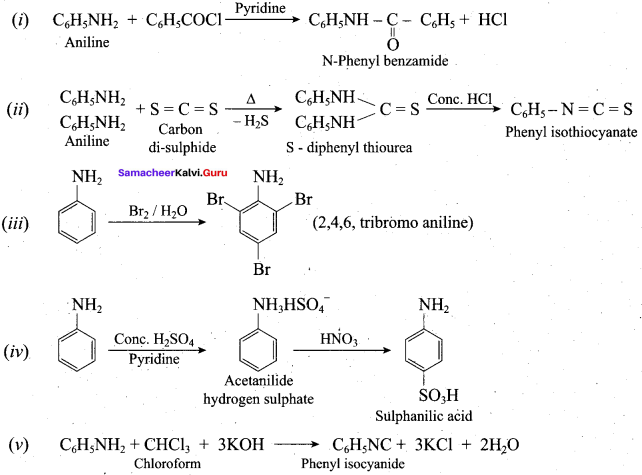
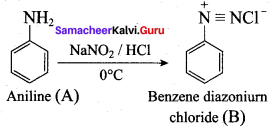
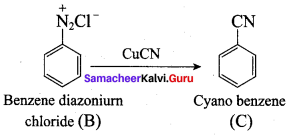
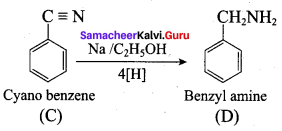
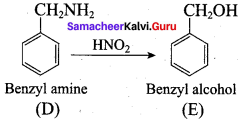
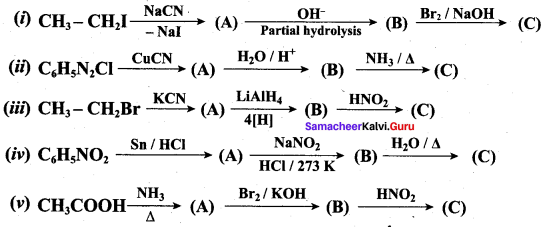
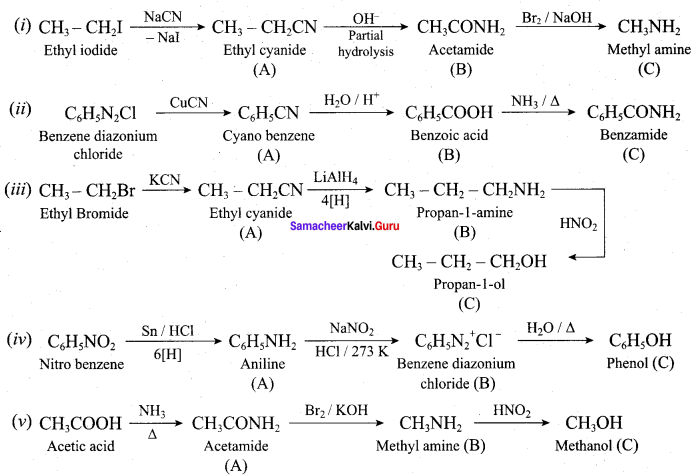
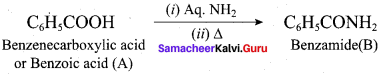
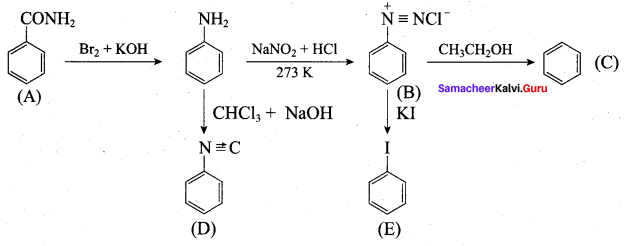
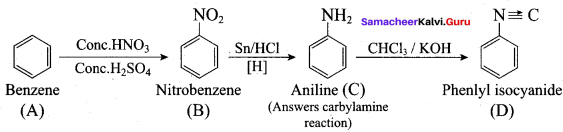
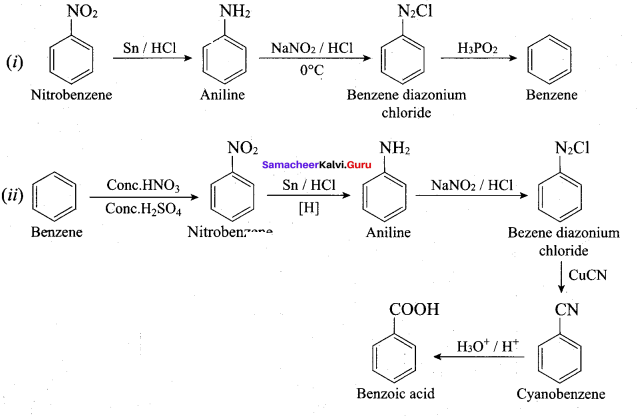
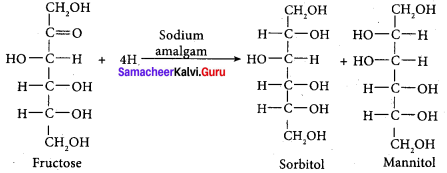
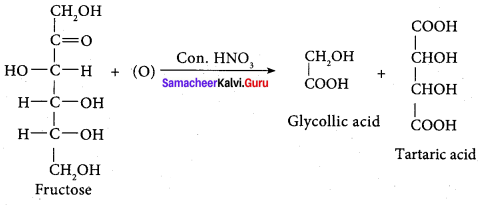
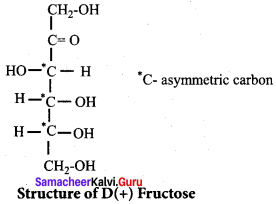
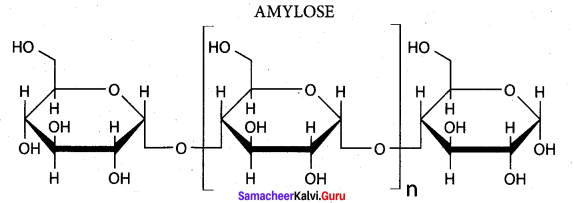
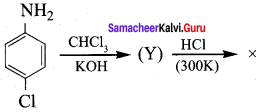
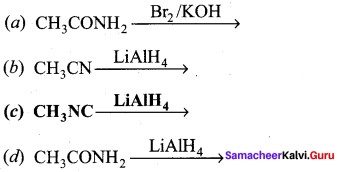
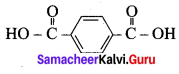 ; Ethylene glycol (Ethane – 1, 2 – diol)
; Ethylene glycol (Ethane – 1, 2 – diol)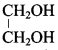
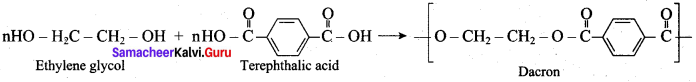

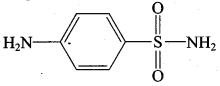

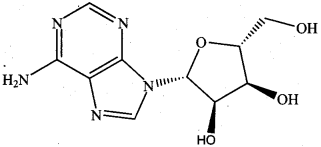
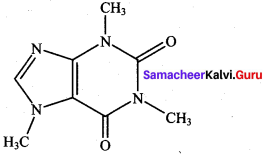
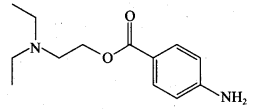


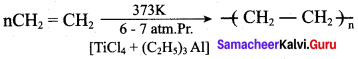
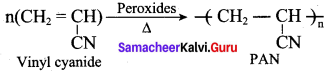
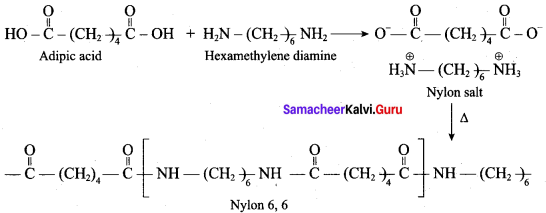
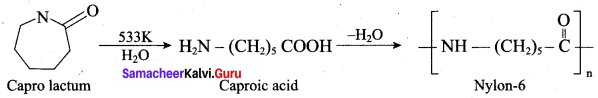
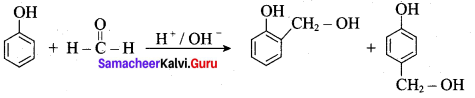
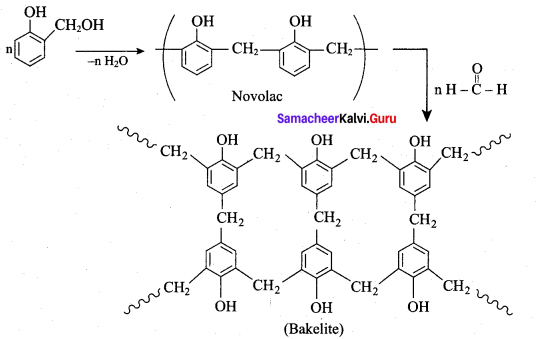
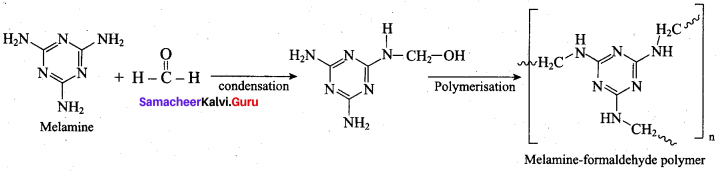
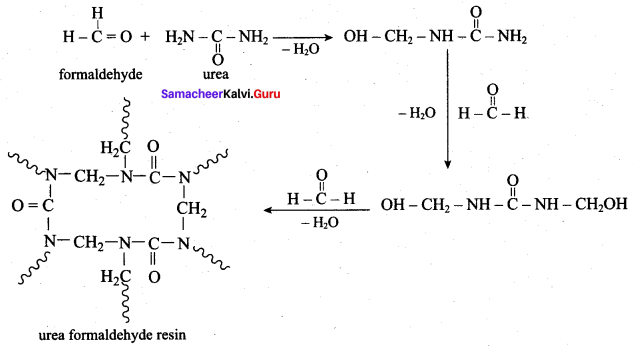
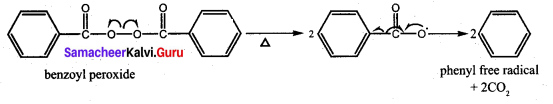
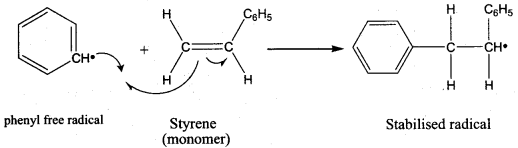
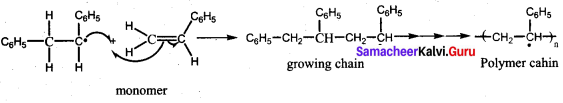
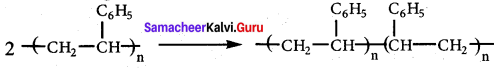
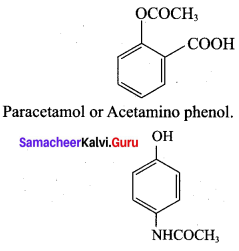
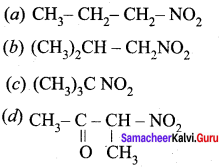
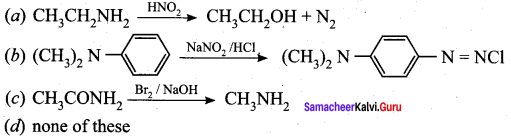
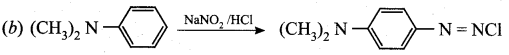
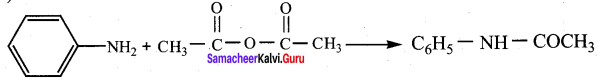
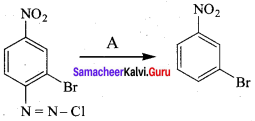
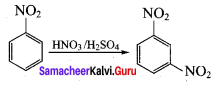

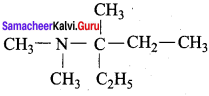 is ………………
is ………………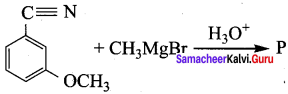
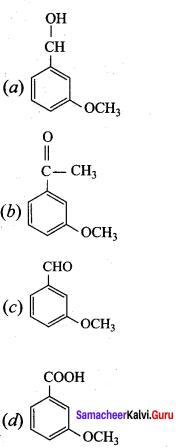
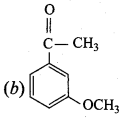
 + Methanoic acid
+ Methanoic acid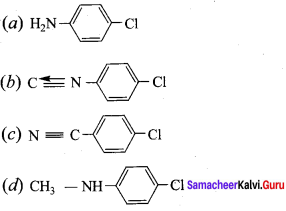
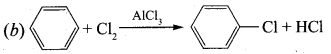
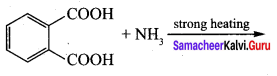
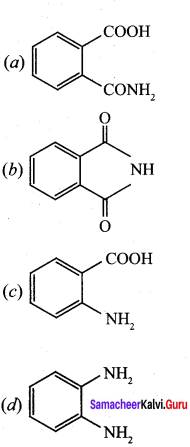
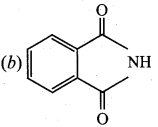
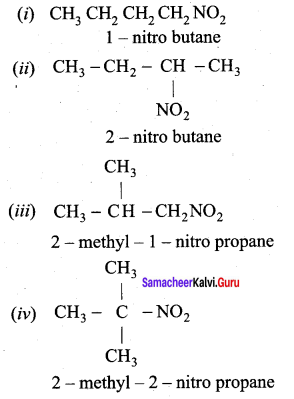
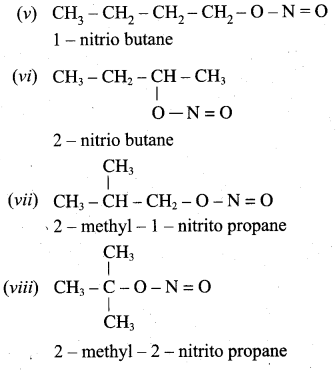
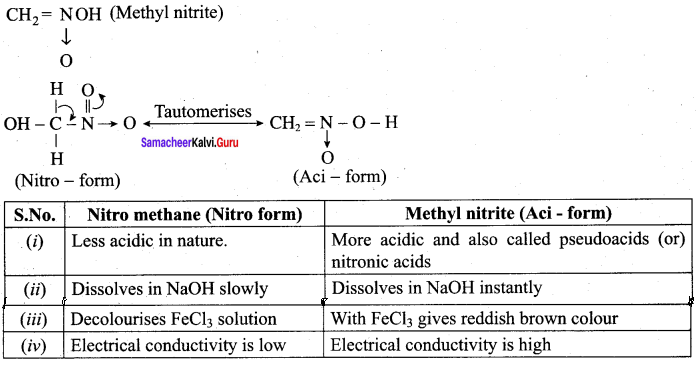
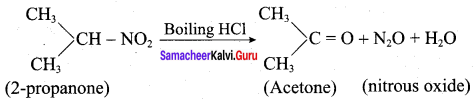
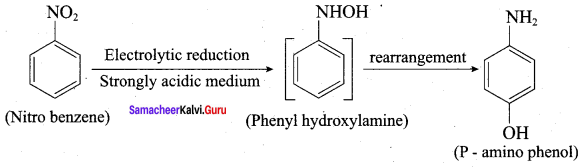
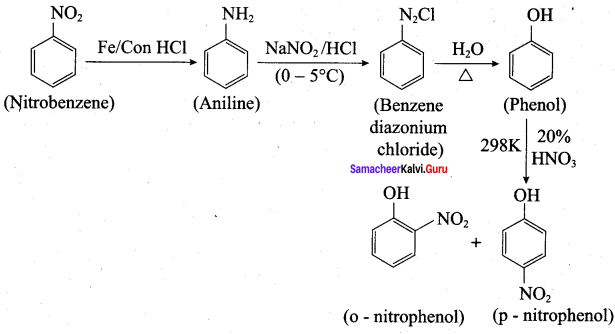
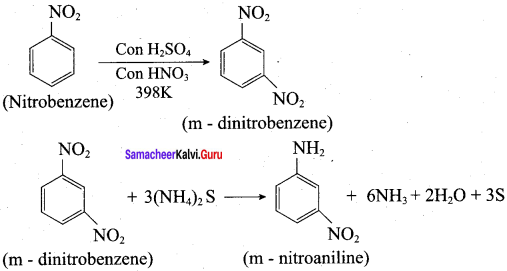
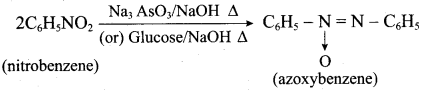
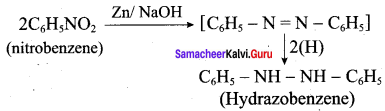
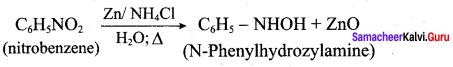
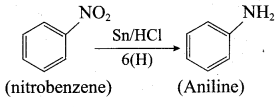
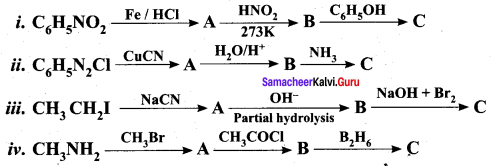
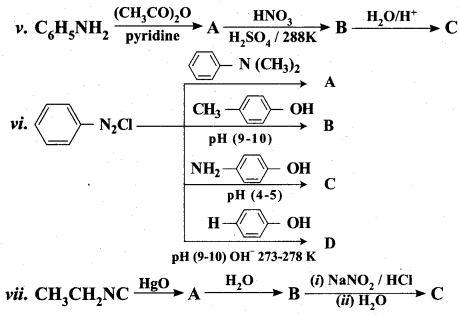
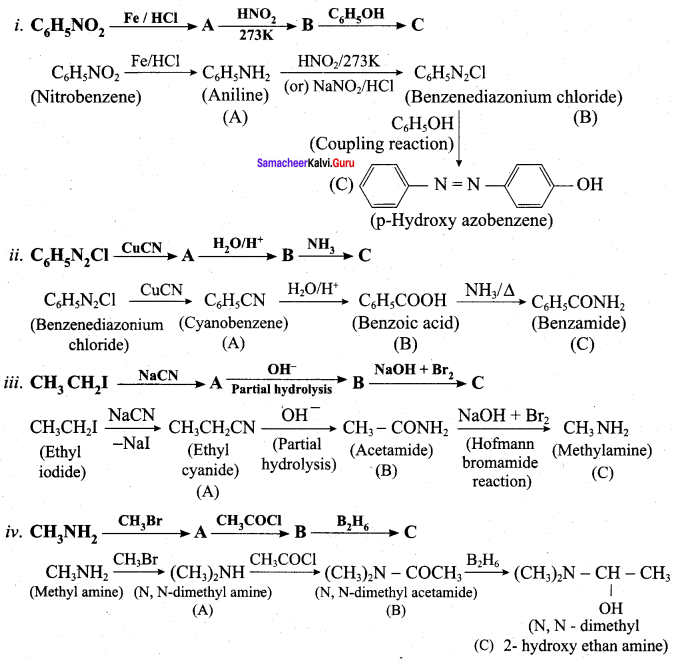
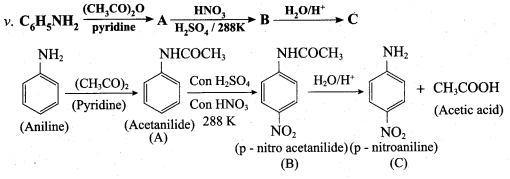
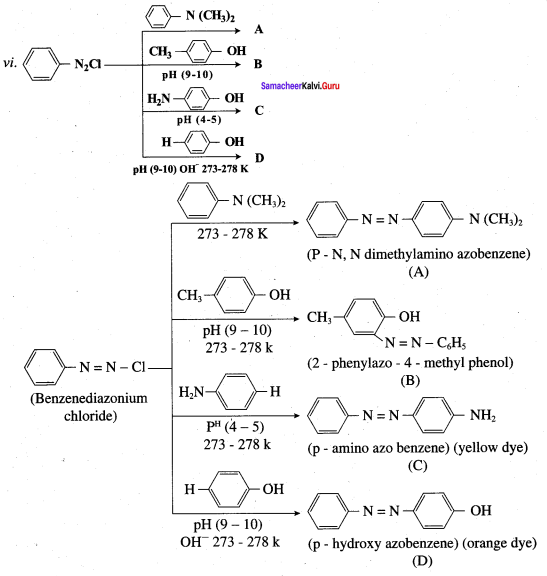
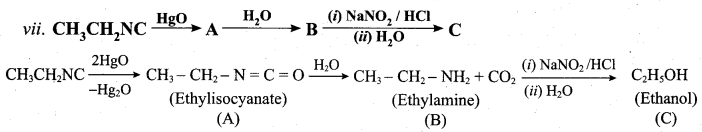
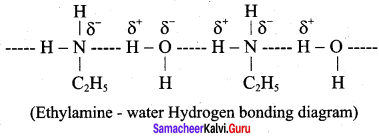
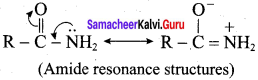
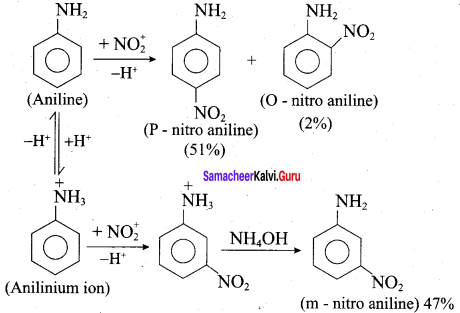
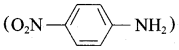
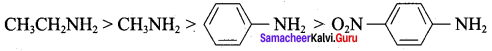
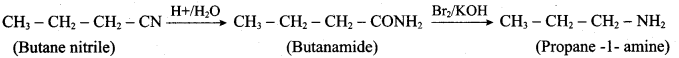
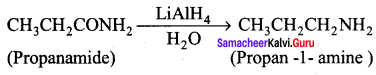
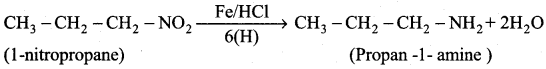
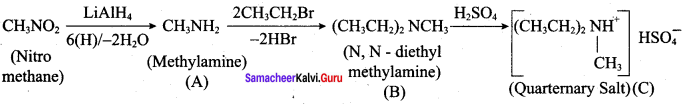


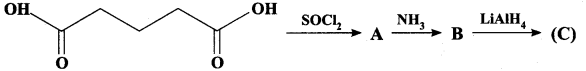
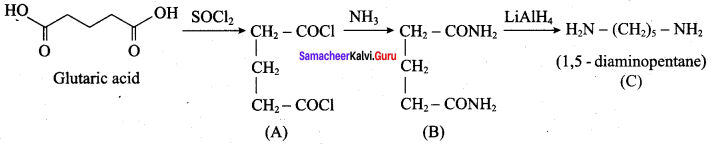
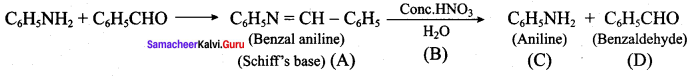
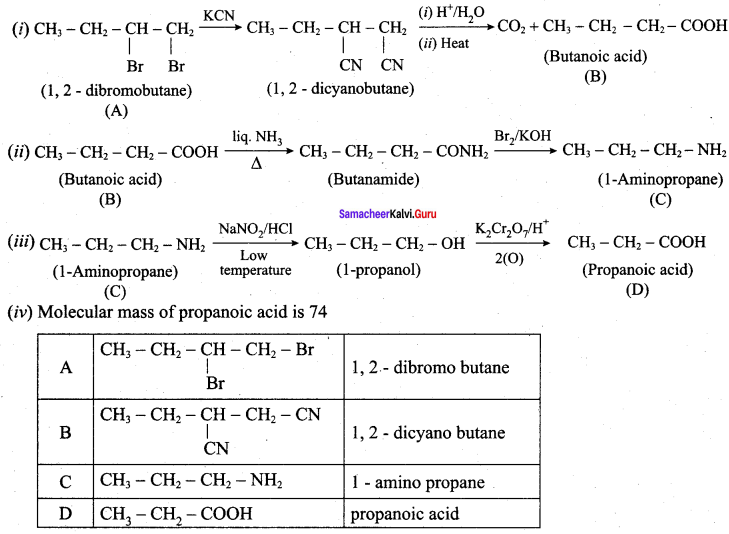
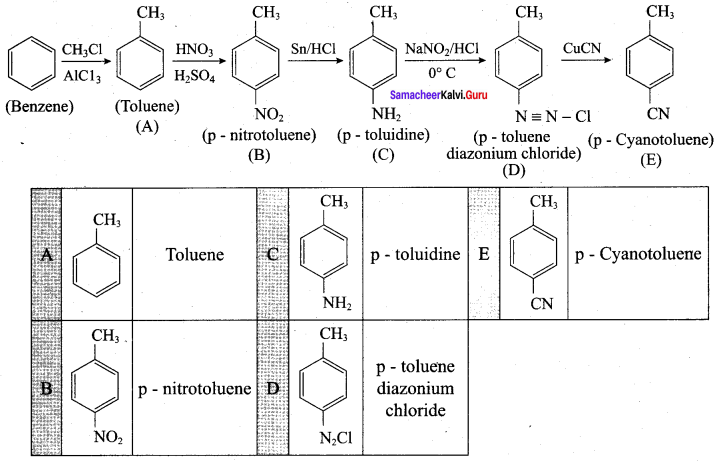
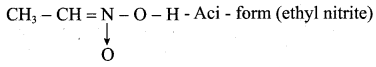

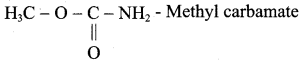
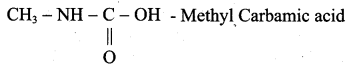
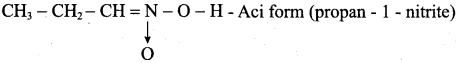


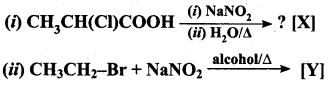
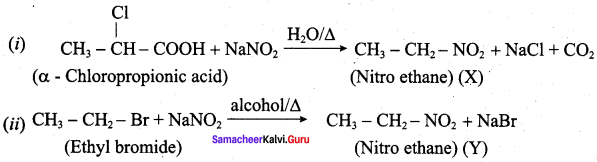
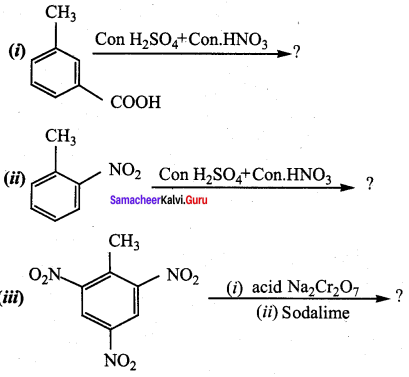
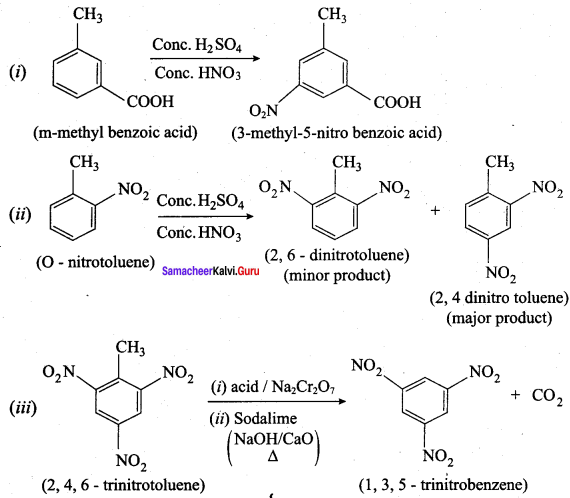
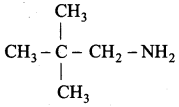
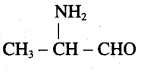
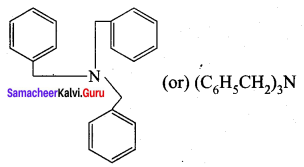
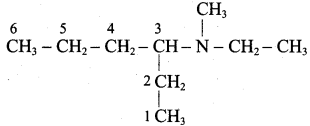
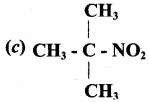
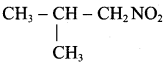 is ………………
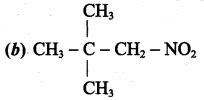
is ………………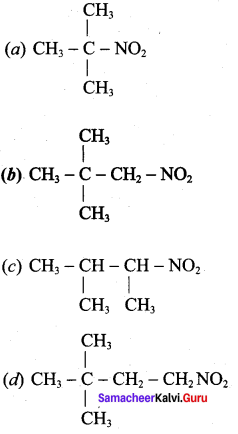
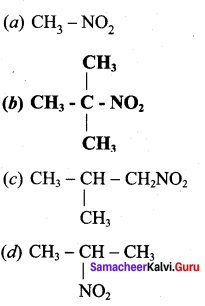
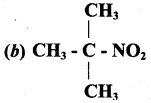
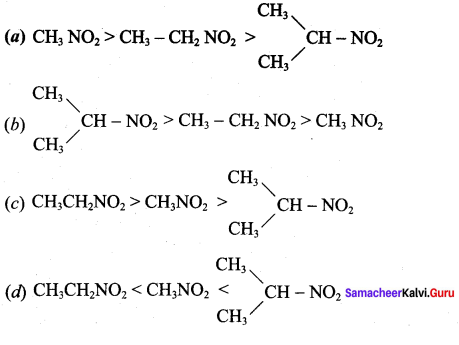
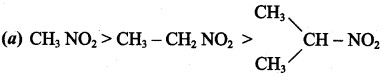
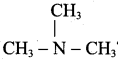
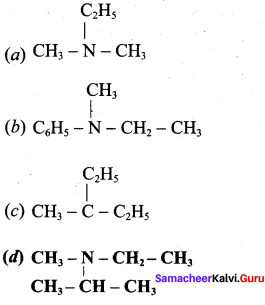
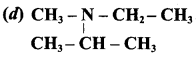
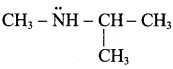 is ……………..
is ……………..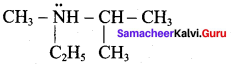
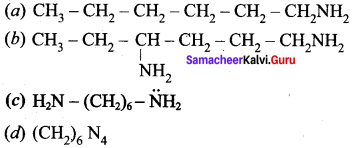
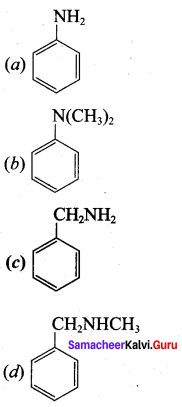
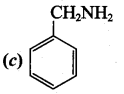
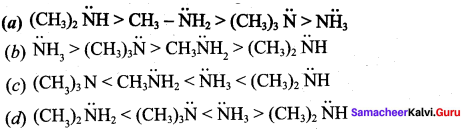
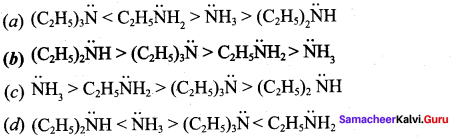
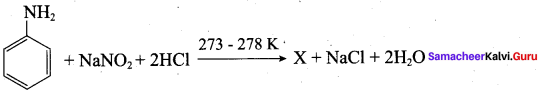
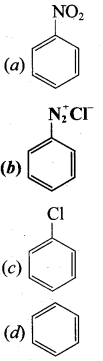
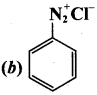
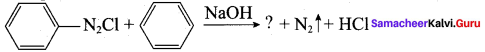
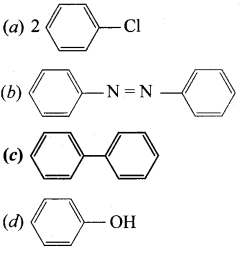
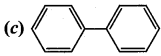
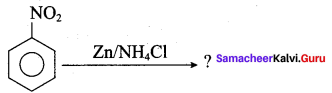
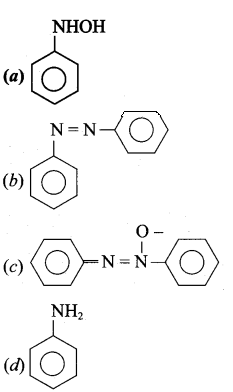
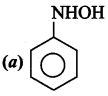
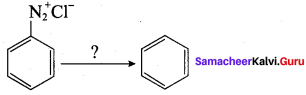
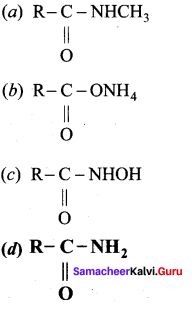
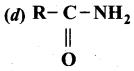
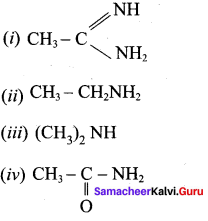
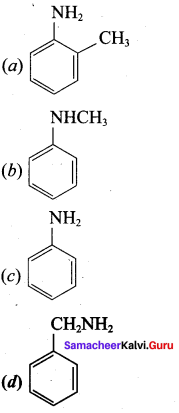
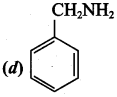
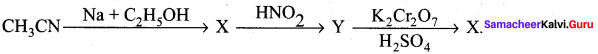

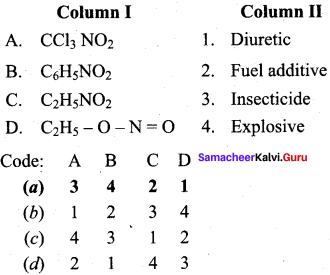
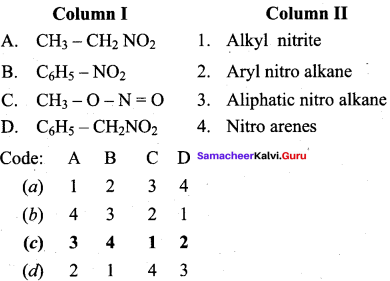
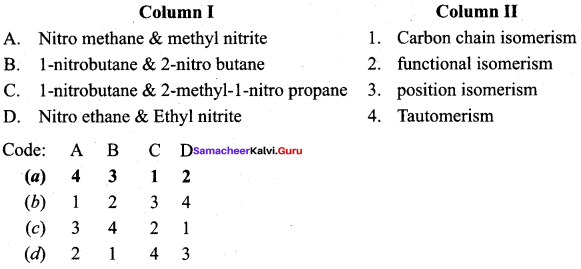
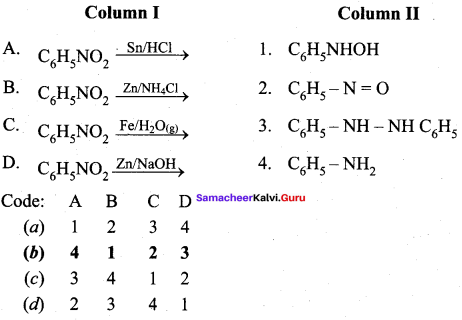
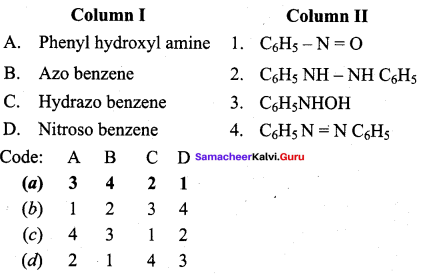
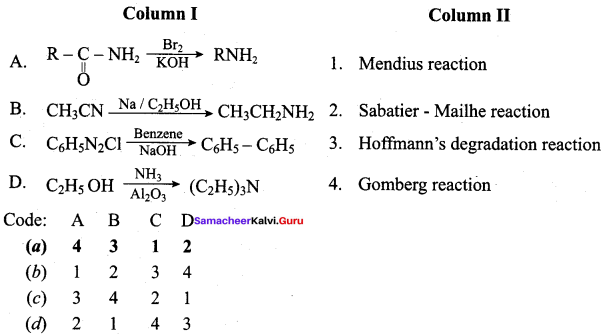
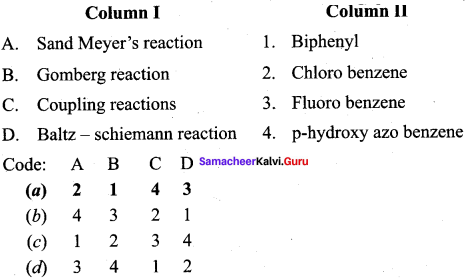
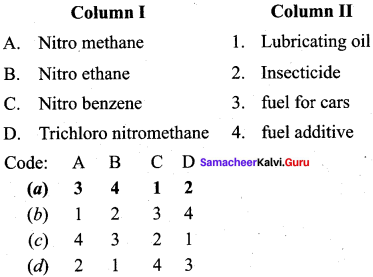
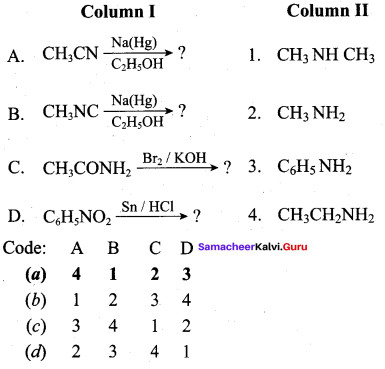

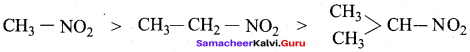
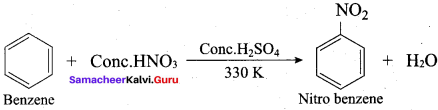
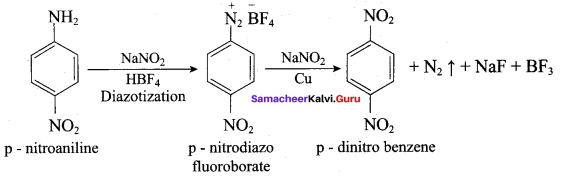
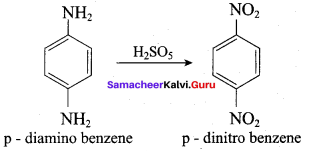
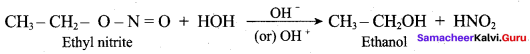
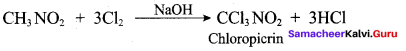

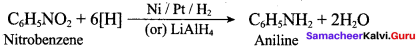
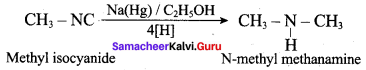
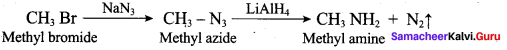
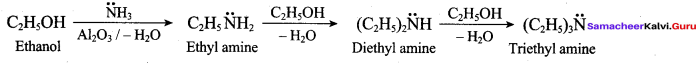
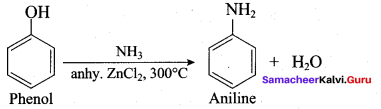
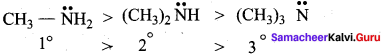

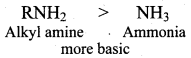
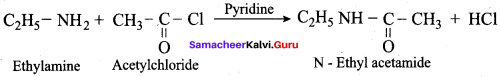
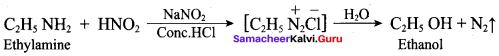
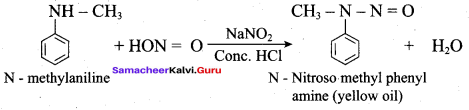
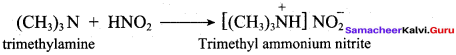
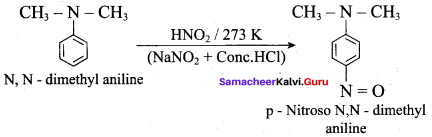
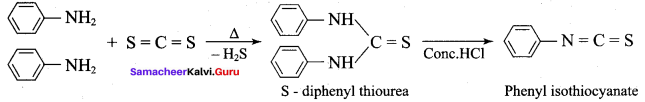
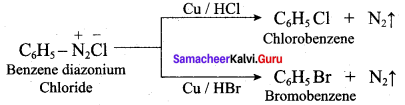
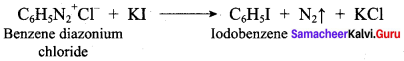
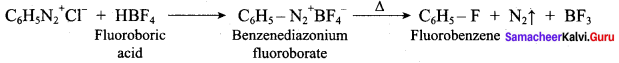
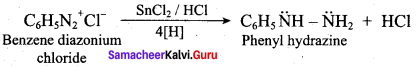
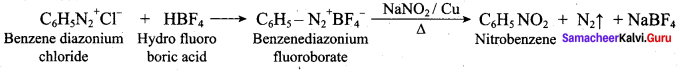
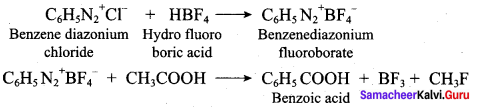
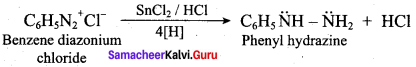

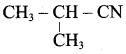
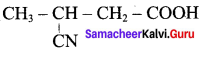
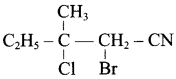
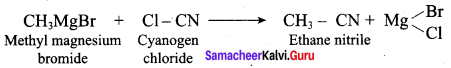
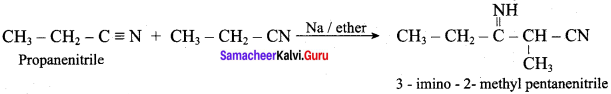
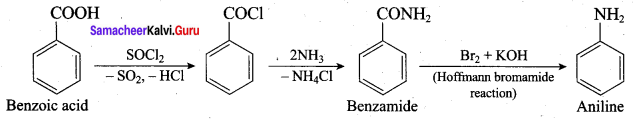
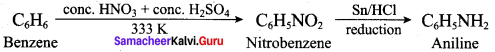
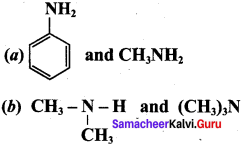
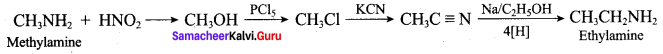
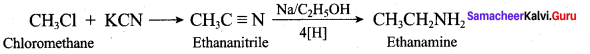
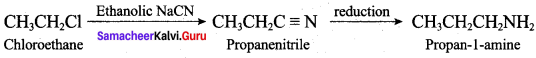
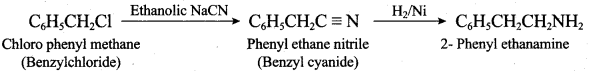
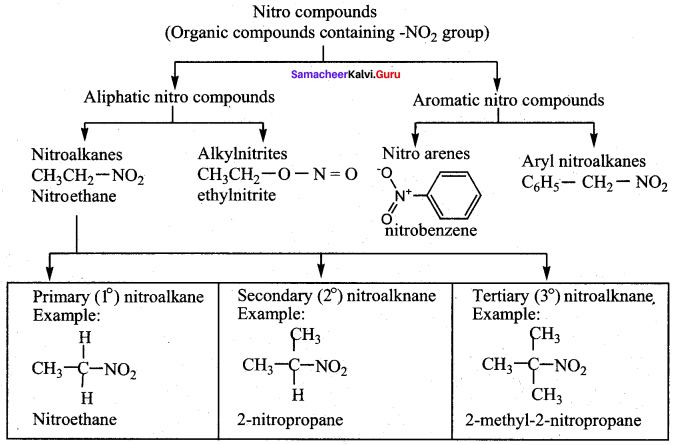
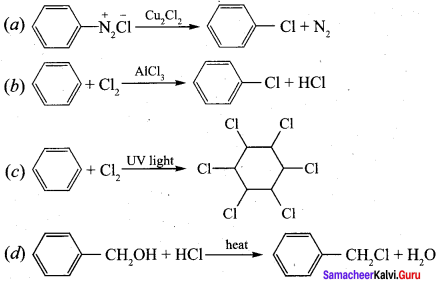
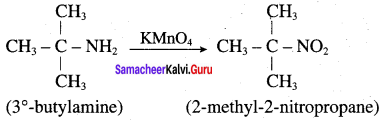
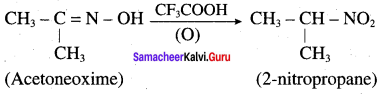
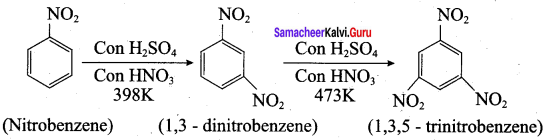
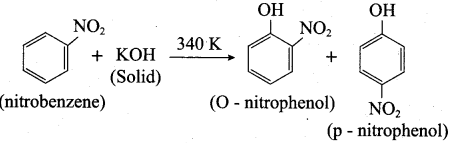
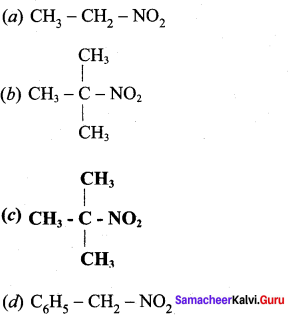
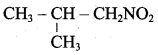 2 – methyl – 1 – nitropropane
2 – methyl – 1 – nitropropane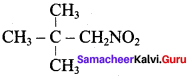 2, 2 – dimethyl – 1- nitropropane
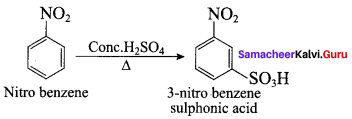
2, 2 – dimethyl – 1- nitropropane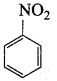 Nitrobenzene
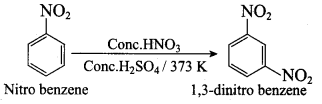
Nitrobenzene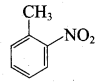 2 – nitro – 1 – methyl benzene
2 – nitro – 1 – methyl benzene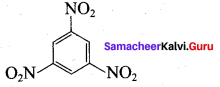 1, 3, 5 – trinitrobenzene
1, 3, 5 – trinitrobenzene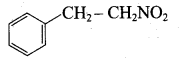 2 – phenyl – 1 – nitroethane
2 – phenyl – 1 – nitroethane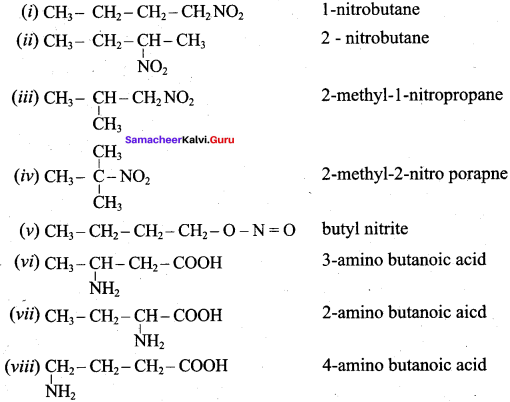
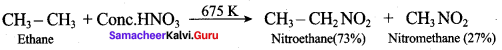
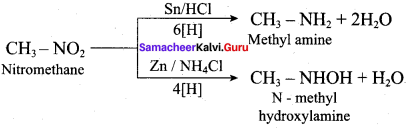
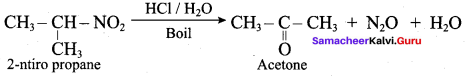
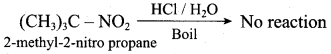
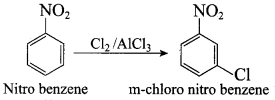
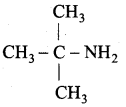
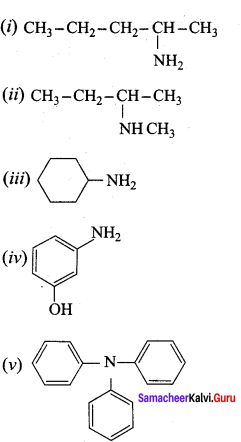
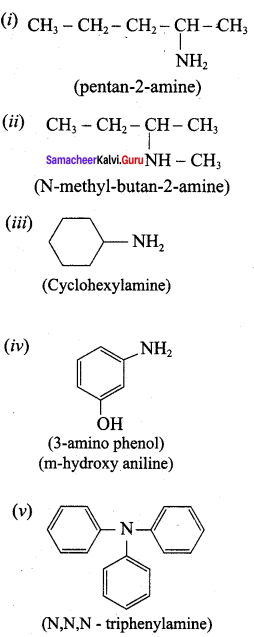
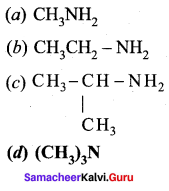
 Propan – 2 – amine
Propan – 2 – amine