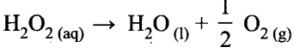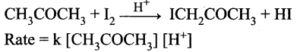Tamilnadu Samacheer Kalvi 12th Chemistry Notes Chapter 10 Surface Chemistry Notes
Surface chemistry: It is the branch of chemistry that deals with the processes occurring at interfaces between phases
Adsorbent: It is the material on which adsorption takes place.
Adsorbate: The adsorbed substacne is called adsorbate.
Interface: The surface of separation of the two phases where the concentration of adsorbed molecule is high is known as interface.
Adsorption: Adsorption is defined as the deposition of molecular species on the surface. The molecular species that get adsorbed on the surfaces is known as adsorbate and the surface on which adsorption occurs is known as adsorbent.
Absorption: It is the physical or chemical process in which atoms, molecules or ions enter some bulk phase (liquid or solid material).
Characteristics of adsorption:
- Adsorption can occur in all interfacial surfaces i.e. the adsorption can occur in between gas-solid, liquid solid, liquid-liquid, solid- solid and gas-liquid.
- Adsorption is always accompanied by decrease in free energy. When AG reaches zero, the equilibrium is attained.
- Adsorption is a spontaneous process.
Desorption: The process of removing a adsorbed substance from the surface is called desorption.
Chemical adsorption: In chemical adsorption gas molecules are held to the surface by formation of chemical bonds.
Physical adsorption: In physical adsorption, physical forces like Vanderwaals force of attraction exists between adsorbent and adsorbate.
Factors affecting adsorption: The extent fo surface adsorption depends on,
- Nature of adsorbent
- Nature of adsorbate
- Pressure
- Concentration at a given temperature
Adsorption isobars: When an amount of adsorption is plotted versus temperature at constant pressure, it is called adsorption isobar.
Adsorption isotherms: A plot between the amount of adsorbate adsorbed and pressure or concentration of adsorbate at constant temperature is called adsorption isotherms.
Catalysis: A catalyst is defined as a substance which alters the rate of chemical reaction without itself undergoing chemical change. The phenomenon which involves the action of a catalyst is called catalysis.
Positive catalysis: In positive catalysis the rate of a reaction is increased by the presence of catalyst.
Negative catalysis: In negative catalysis the rate of reaction is decreased by the presence of catalyst.
Homogeneous catalysis: In this catalysis reaction, the reactants, products and catalyst are present in the same phase.
Heterogeneous catalysis: In this catalysis reaction, the catalyst is present in a different phase (i.e.) it is not present in same phase as that of reactants or products.
Characteristics of catalysts:
- For a chemical reaction, catalyst is needed in very small quantity. Generally, a pinch of catalyst is enough for a reaction in bulk.
- There may be some physical changes, but the catalyst remains unchanged in mass and chemical composition in a chemical reaction.
- A catalyst itself cannot initiate a reaction. It means it cannot start a reaction which is not taking place. But, if the reaction is taking place in a slow rate it can increase its rate.
- A catalyst is highly effective at a particular temperature called as optimum temperature.
- Presence of a catalyst generally does not change the nature of products.
Promoters: In a catalysed reaction, the presence of a certain substance increases the activity of a
catalyst. Such a substance is called a promoter.
Catalyst poison: Certain substances when added to a catalysed reaction decreases or completely destroys the activity of catalyst and they are often known as catalytic poisons.
Auto catalysis: In certain reactions one of the products formed acts as a catalyst to the reaction.
Initially the rate of reaction will be very slow but with the increase in time the rate of reaction increases.
Active centres: The surface of a catalyst is not smooth. It bears steps, cracks and comers. Hence the atoms on such locations of the surface are co-ordinatively unsaturated. So, they have much residual force of attraction. Such sites are called active centres. So, the surface carries high surface free energy.
Enzyme Catalysis: Enzymes are complex protein molecules with three dimensional structures. They catalyse the chemical reaction in living organism. They are often present in colloidal state and extremely specific in catalytic action. Each enzyme produced in a particular living cell can catalyse a particular reaction in the cell.
Zeolite Catalysis: Zeolites are microporous, crystalline, hydrated, alumino silicates, made of silicon and aluminium tetrahedra. There are about 50 natural zeolites and 150 synthetic zeolites. As silicon is tetravalent and aluminium is trivalent, the zeolite matrix carries extra negative charge. To balance the negative charge, there are extra framework cations for example, H+ or Na+ ions.
Phase transfer catalysis: Phase transfer catalyst to facilitate transport of a reactant in one solvent to the other solvent where the second reactant is present. As the reactants are now brought together they rapidly react and form the product.
Nano catalysis: Nano materials such a metallic nano particles, metal oxides, etc., are used as catalyst in many chemical transformation, Nano catalysts carry the advantages of both homogeneous and heterogeneous catalysis.
Colloids: It is a homogeneous mixture of two substances in which one substance (smaller proportion) is dispersed in another substance (Large proportion).
Dispersed phase and Dispersed medium: In a colloidal, the substance present in larger amount is called dispersing medium and the substance present in less amount is called dispersed phase.
Preparation of Colloids: In general, colloidals are prepared by the following methods,
- Dispersion methods: In this method larger particles are broken to colloidal dimension.
- Condensation method: In this method, smaller atom or molecules are converted into larger colloidal sized particles.
Peptisation: By addition of suitable electrolytes precipitated particles can be brought into colloidal state. The process is termed as peptisation and the electrolyte added is called peptising or dispersing agent.
Tyndall effect: When light is passed through colloidal solution, it is scattered in all directions. This effect is called Tyndall effect.
Brownian movement: The colloidal sol particles are continuously bombard with the molecules of dispersion medium and hence they follow a zigzag random, continuous movement.
Helmholtz double layer: The surface of colloidal particle adsorbs one type of ion due to preferential adsorption. This layer attracts the oppositely charged ions in the medium and hence at the boundary separating the two electrical double layers are setup. This is called as Helmholtz electrical double layer.
Electrophoresis: The migration of sol particles under the influence of electric field is called Electrophoresis.
Electro osmosis: The movement of dispersion medium under the influence of electric potential is called Electro osmosis.
Emulsion: They are colloidal solution in which a liquid is dispersed in an another liquid.
Type of Emulsion: Generally there are two types of emulsions.
(i) Oil in water (O/W) (ii) Water in oil (W/O)
Deemulsification: Emulsion can be separated into two separate layers. This process is called Deemulsification.
Cortrell’s precipitator: Carbon dust in air is solidified by cortrell’s precipitator. In it, a high potential difference of about 50,000 V is used. Th e charge on carbon is neutralized and solidified. Thus the air is free from carbon particles.
Classifications of colloids based on the physical state of dispersed phase and dispersion medium:
| Dispersion medium | Dispersed phase | Name of the colloid |
Examples |
| Gas | Liquid | Liquid Aerosol | Fog, Aerosol spray |
| Gas | Solid | Solid Aerosol | Smoke, Air pollutants like fumes, dust. |
| Liquid | Gas | Foam | Whipped cream, Shaving cream, Soda water, Froth. |
| Liquid | Liquid | Emulsion | Milk, Cream, Mayonnaise |
| Liquid | Solid | Sol | Inks, Paints,colloidal gold. |
| Solid | Gas | Solid foam | Pumice stone, Foam rubber bread. |
| Solid | Liquid | Gel | Butter, cheese |
| Solid | Solid | Solid sol | Pearls, opals coloured glass alloys colloidal dispersed eutelics. |
Shape of colloidal particles:
| Colloidal Particles |
Shapes |
| AS2S3 | Spherical |
| Fe(OH)3 sol (blue gold sol) | Disc or plate like |
| W3O5 sol (tungstic acid sol) | Rod like |
Example of charges of sols detected by Electrophoresis are given below:
| Positively charge colloids | Negatively charge colloids |
| Ferric hydroxide | Ag, Au & Pt |
| Aluminium hydroxide | Aresenic sulphide |
| Basic dyes | Clay |
| Haemoglobin | Starch |