Tamilnadu Samacheer Kalvi 12th Chemistry Notes Chapter 4 Transition and Inner Transition Elements Notes
Position of d-block elements: Group – 3 to group – 12 of the modem periodic table is the position of d-block elements.
Electronic configuration: [Noble gas] (n – l)d1-10 ns1-2
Metallic behaviours: Except Cu, Ag, Au all are hard. Most of them are hexagonal close packed, cubic close packed (or) body centred cubic which are the characteristics of true metals.
Variation (or) trend in properties of d-block elements:
| Property | Across the period |
Down the group |
| 1. Melting point | First increases and reaches maximum value then decreases | Decreases |
| 2. Atomic and ionic size | Generally decreases, but in 3d series, atomic radius decreases Sc to V and then upto Cu, remains the same. | Generally increases |
| 3. Ionization enthalpy | Increases | Decreases |
| 4. Oxidation state | Except Sc, all exhibit variable oxidation states generally increase and then decrease. | Decreases |
Standard electrode potentials: It is the value of the standard emf of a cell in which molecular hydrogen under standard pressure ( latm) and temperature (273K) is oxidised to solvated protons at the electrode.
Magnetic properties: Most of the compounds of transition elements are paramagnetic.
Magnetic moment: μ = \(g \sqrt{S(S+1)} \mu_{B}\)
where g = 2, S is the total spin quantum number of unpaired electrons and pB is Bohr Magneton.
Catalytic property: Most of the transition metals and their compounds act as catalyst.
Alloy formation: An alloy is formed by blending a metal with one or more other metals.
Formation of interstitial compounds: It is a compound that is formed when small atoms like hydrogen, boron, carbon or nitrogen are trapped in the interstitial holes in a metal lattice.
Formation of complex: Transition elements have a tendency to form complex compounds with a species that has an ability to donate an electron pair to form a coordinate covalent bond.
K2Cr2O7: Potassium dichromate is an orange red crystalline solid.
Structure of dichromate ion:
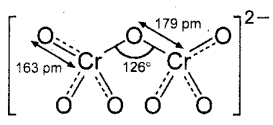
Potassium permanganate -KMnO4: It is a dark purple crystals.
Structure of permanganate ion:
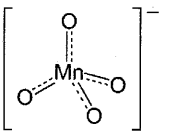
Baeyer’s reagent: Cold, dilute alkaline KMnO4
f-block elements: 1. Lanthanoids 2. Actinoids
Lanthanoids and Actinoids differences:
| Lanthanoids | Actinoids |
| Electronic configuration [Xe] 4f1-14 5d0-1 6s2 | Electronic configuration [Rn] 5f1-14 6d0-27s2 |
| Common oxidation state is +3 | Common oxidation state is +4 |
| Differentiating electron eneters 4f orbital | Differentiating electron eneters in 5f orbital |
| Binding energy of 4f orbitals are higher | Binding energy of 5f orbitals are lower |
| They show less tendency to form complexes | They show greater tendency to form complexes |
| Most of the lanthanoids are colourless | Most of the actinoids are coloured. E.g : U3+ (red), U4+ (green), UO22+(yellow) |
| They do not form oxo cations | They do form oxo cations such as UO22+, NpO22+ etc. |
| Besides +3 oxidation states lanthanoids show +2 and +4 oxidation states in few cases. | Besides +3 oxidation states actinoids show higher oxidation states such as +4, +5, +6 and +7. |