Tamilnadu Samacheer Kalvi 11th Chemistry Notes Chapter 14 Haloalkanes and Haloarenes Notes
Haloalkanes – When one or more hydrogen atoms of aliphatic hydrocarbons are replaced by a halogen atom, the resultant compounds are called Haloalkanes.
Haloarene – When one or more hydrogen atoms of aromatic hydrocarbons are replaced by a halogen atom , the resultant compounds are called Haloarenes.
R-X – Mono halogen derivatives of alkanes are called haloalkanes. They are classified as 1° (Primary), 2° (secondary) and 3° (Tertiary) haloalkanes.
Nature of C-X bond in haloalkanes – Carbon halogen bond is a polar bond as halogens are more electronegative than carbon.
Ammonolysis – Reaction with alcoholic ammonia:
Ambident Nucleophile – Nucleophiles such as cyanide and nitrite ion which can attack the nucleophilic centre from two sides are called ambident nucleophiles..
SN2 – Bimolecular nucleophilic substitution reactions.
SN1– Unimolecular nucleophilic substitution reactions.
E2 reaction – Bimolecular elimination reactions.
E1 reaction – Unimolecular elimination reactions.
Organometallic compounds – They are organic compounds in which there is a direct carbon-metal bond, e.g., CH3MgI Methyl magnesium iodide.
Haloarene – C6H5-X: In this type of compounds atoms is directly attached to benzene ring.
Resonance Structure of Halobenzene –
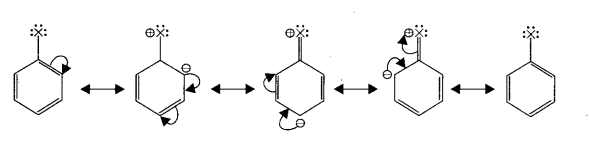
Polyhalogen compounds – Carbon compounds containing more than one halogen atoms are called polyhalogen compounds.
Gemdihalide – If two halogen atoms are attached to one carbon atom, it is called gem dihalide, eg., CH3CHCl2-Ethylidene chloride.
Vicinal dihalide – If two halogen atoms are attached to first two carbon atoms, it is called vicinal dihalide.
CHCl3 – It is chloroform.
CCl4 – It is tetrachloromethane (or) Carbontetrachloride.
Freops – (CFC’s) The chlorofluoro derivatives of methane and ethane are called freons.
DDT – It stands for p,p’ – dichloro diphenyl trichloroethane. It is an organic pesticide.