Are you searching for the Samacheer Kalvi 12th Chemistry Chapter Wise Solutions PDF? Then, get your Samacheer Kalvi 12th Chapter Wise Solutions PDF for free on our website. Students can Download Chemistry Chapter 8 Ionic Equilibrium Questions and Answers, Notes Pdf, Samacheer Kalvi 12th Chemistry Solutions Guide Pdf helps you to revise the complete Tamilnadu State Board New Syllabus and score more marks in your examinations.
Tamilnadu Samacheer Kalvi 12th Chemistry Solutions Chapter 8 Ionic Equilibrium
All concepts are explained in an easy way on our website. So, students can easily learn and practice Tamilnadu State Board 12th Chemistry Chapter 8 Ionic Equilibrium Question and Answers. You can enjoy the digitized learning with the help of the Tamilnadu State Board Chemistry Online Material.
Samacheer Kalvi 12th Chemistry Chapter 8 Ionic Equilibrium Textual Evaluation Solved
Samacheer Kalvi 12th Chemistry Ionic Equilibrium Multiple Choice Questions
I. Choose the correct answer.
12th Chemistry Ionic Equilibrium Question 1.
Concentration of the Ag+ ions in a saturated solution of Ag2C2O4 is 2.24 x 10-4 mol L-1 solubility product of Ag2C2O4 is ………………
(a) 2.42 x 10-8 mol3 L-3
(b) 2.66 x 10-12 12 mol3 L-3
(c) 45 x 10-11 mol3 L-3
(d) 5.619 x 10-12 mol3 L-3
Answer:
(d) 5.619 x 10-12 mol3 L-3
Ag2C2O4 2Ag+ + C2 O42-
[Ag+] = 2.24 x 10-4 mol L-1
![]()
= 1.12 x 10-4 mol L-1
Ksp = [Ag+]2 [C2O42-]
=(2.24 x 10-4 mol L-1)2 (1.12 x 10-4 mol L-1)
=5.619 x 10-12 mol3 L-3
12th Chemistry Chapter 8 Book Back Answers Question 2.
Following solutions were prepared by mixing different volumes of NaOH of HCl different concentrations.
(i) 60 mL \(\frac { M }{ 10 }\) HCI + 40 mL \(\frac { M }{ 10 }\) NaOH
(ii) 55 mL \(\frac { M }{ 10 }\) HCl + 45 mL \(\frac { M }{ 10 }\) NaOH
(iii) 75 mL \(\frac { M }{ 5 }\) HCI +25 mL \(\frac { M }{ 5 }\) MNaOH
(iv) 100 mL \(\frac { M }{ 10 }\) HCI+ 100 mL \(\frac { M }{ 10 }\) NaOH
pH of which one of them wilt be equal to 1?
(a) (iv)
(b) (i)
(c) (ii)
(d) (iii)
Answer:
(d) (iii) 75 mL \(\frac { M }{ 5 }\) HCI + 25 mL \(\frac { M }{ 5 }\) NaOH
No of moles of HCl = 0.2 x 75 x 10-3 = 15 x 10-3
No of moles of NaOH = 0.2 x 25 x 10-3 = 5 x 1o-3
No of moles of HCl after mixing = 15 x 10-3 – 5 x 10-3
∴ Concentration of HCl

for (iii) solution, pH of 0.1 M HCI = – 1og10 (0.1) = 1.
12th Chemistry 8th Lesson Book Back Answers Question 3.
The solubility of BaSO4 in water is 2.42 x 10-3 gL-1 at 298K. The value of its solubility product
(Ksp) will be …………………..
(Given molar mass of BaSO4 = 233g mol-1)
(a) 1.08 x 10-14 mol2L2
(b) 1.08 x 10-12 mol2L2
(c) 1.08 x 10-10 mol2 L2
(d) 1.08 x 10-8 mol2L-2
Answer:
(c) 1.08 x 10-10 mol2 L2
BaSO4 \(\rightleftharpoons\) Ba2+ + SO42-
Ksp = (s) (s)
Ksp = (s)2

Ionic Equilibrium Questions And Answers Pdf Question 4.
pH of a saturated solution of Ca(OH)2 is 9. The Solubility product (K) of Ca(OH)2 ………………..
(a) 0.5 x 10-15
(b) 0.25 x 10-10
(c) 0.125 x 10-15
(d) 0.5 x 10-10
Answer:
(a) 0.5 x 10-15
Ca(OH)2 \(\rightleftharpoons\) Ca2+ + 2OH–
Given that pH = 9
pOH = 14 – 9 = 5
[p0K = – 1og10[OH–]]
[OH–] = 10-pOH
[OH–] =10-5M
Ksp = [Ca2+] [OH–]2
![]()
=0.5
Class 12 Ionic Equilibrium Question 5.
Conjugate base for bronsted acids H2O and HF are ………………
(a) OH– and H2FH+, respectively
(b) H3O+ and F–, respectively
(c) OH– and F–, respectively
(d) H3O+ and H2F+, respectively
Answer:
(c) OH– and F–, respectively

∴ Conjugate bases are OH– and F– respectively
Ionic Equilibrium Notes Pdf Question 6.
Which will make basic buffer?
(a) 50 mL of 0.1M NaOH + 25mL of 01M CH3COOH
(b) 100 mL of 0.1M CH3COOH + 100 mL of 0.1M NH4OH
(c) 100 mL of 0.1M HCI + 200 mL of 0.1M NH4OH
(d) 100 mL of 0.1M HCI + 100 mL of O.1 M NaOH
Answer:
(c) 100 mL of 0.1M HCI + 200 mL of 0.1M NH4OH
Basic buffer is the solution which has weak base and its salt
![]()
Samacheer Kalvi Guru 12th Chemistry Question 7.
Which of the following fluro – compounds is most likely to behave as a Lewis base?
(a) BF3
(b) PF3
(c) CF4
(d) SiF4
Answer:
(b) PF3
BF3 → electron deficient → Lewis acid
PF3 → electron rich → Lewis base
CF4 → neutral → neither lewis acid nor base
SiF4 → neutral → neither lewis acid nor base
Samacheer Kalvi Class 12 Chemistry Solutions Question 8.
Which of these is not likely to act as lewis base?
(a) BF3
(b) PF3
(c) CO
(d) F–
Answer:
(a) BF3
BF3 → electron deficient → Lewis acid
PF3 → electron rich → Lewis base
CO → having lone pair of electron → Lewis base
F– → unshared pair of electron → lewis base
Ionic Equilibrium Notes Question 9.
What is the decreasing order of strength of bases?
OH–, NH2–, H – C = C– and CH3 – CH2–
(a) OH– > NH2– > H – C = C > CH3 – CH2–
(b) NH2– > OH– > CH3 – CH2– > H – C = C–
(c) CH3 – CH2–, > NH2– > H – C = C– > OH–
(d) OH– > H – C = C > CH3 – CH2– > NH2–
Answer:
(c) CH3 – CH2–, > NH2– > H – C = C– > OH–
Acid strength decreases in the order
HOH > CH = CH > NH3 > CH3CH3
Its conjucate bases arc in the reverse order
CH3 – CH2– > NH2– > H – C = C > OH–
Ionic Equilibrium Question 10.
The aqueous solutions of sodium formate, anilinium chloride and potassium cyanide are respectively
(a) acidic, acidic, basic
(b) basic, acidic, basic
(c) basic, neutral, basic
(d) none of these
Answer:
(b) basic, acidic, basic

Class 12 Chemistry Chapter 8 Notes Question 11.
The percentage of pyridine (C5H5N) that forms pyridinium ion (C5H5NH) in a 0.10M aqueous pyridine solution (Kb for C5H5N = 1.7 x 10-9) iS ……………..
(a) 0.006%
(b) 0.013%
(c) 0.77%
(d) 1.6%
Answer:
(b) 0.013%

Percentage of dissociation
= \(\sqrt { 1.7 }\) x 10-4 x 100 = 1.3 x 10-2 = 0.013%
Question 12.
Equal volumes of three acid solutions of pH 1,2 and 3 are mixed in a vessel. What will be the H+ ion concentration in the mixture?
(a) 37 x 10-2
(b) 10-6
(c) 0.111
(d) none of these
Answer:
(a) 3.7 x 10-2
pH = – log10 [H+]
[H+] = 10-pH
Let the volume be x mL
V1M1 + V2M2 + V3M3 = VM
x mL of 10-1M + x mL of 10-2M + x mL of 10-3 M
= 3 x mL of [H+]
= 3 x mL of [H+]
[H+] =
![]()
= 0.037 = 3.7 x 10-2
Question 13.
The solubility of AgCl (s) with solubility product 1.6 x 10-10 in 0. 1 M NaCl solution would be ………….
(a) 1.26 x 10-5 M
(b) 1.6 x 10-9 M
(c) 1.6 x 10-11 M
(d) Zero
Answer:
(b) 1.6 x 10-9 M
AgCl (s) \(\rightleftharpoons\) Ag+(aq) + Cl–(aq)
![]()
Ksp = 1.6 x 10-10
Ksp = [Ag+][Cl–]
K = (s) (s+0.1)
0.1 >>s

Question 14.
If the solubility product of lead iodide is 3.2 x 10-8, its solubility will be …………..
(a) 2 x 10-3M
(b) 4 x 10-4 M
(c) l.6 x 10-5 M
(d) 1.8 x 10-5 M
Answer:
(a) 2 x 10-3M
PbI2 (s) → Pb2+ (aq) + 2I– (aq)
Ksp = (s) (2s)2
3.2 x 10-8 = 4s3
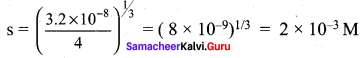
Question 15.
Using Gibb’s free energy change, ∆G0 = 57.34 KJ mol-1, for the reaction, X2Y(g) \(\rightleftharpoons\) 2X+ + Y2-(aq), calculate the solubility product of X2Y in water at 300K (R = 8.3 J K-1 Mol-1) ……………….
(a) 10-10
(b) 10-12
(c) 10-14
(d) can not be calculated from the given data
Answer:
(a) 10-10
57.34 KJ mol-1 = – 2.303 x 8.3 JK-1 mol-1 x 300K log Ksp
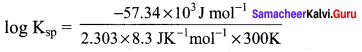
log10Ksp = -10
∴ Ksp = 10-10
∆G0 = – 2.303 RT log Keq
X2Y(s) \(\rightleftharpoons\) 2X+(aq) +Y2-(aq)

Keq = [x+]2[Y2-] ( X2Y(s) = 1)
Keq = K
Question 16.
MY and NY3, are insoluble salts and have the same Ksp values of 6.2 x 10-13 at room temperature. Which statement would be true with regard to MY and NY3?
(a) The salts MY and NY3 are more soluble in O.5 M KY than in pure water
(b) The addition of the salt of KY to the suspension of MY and NY3 will have no effect on
(c) The molar solubities of MY and NY3 in water are identical
(d) The molar solubility of MY in water is less than that of NY3
Answer:
(d) The molar solubility of MY in water is less than that of NY3
Addition of salt KY (having a common ion Y–) decreases the solubility of MY and NY3 due to common ion effect. Option (a) and (b) are wrong.
For salt MY, MY \(\rightleftharpoons\) M+ + Y–
Ksp = (s) (s)
6.2 x 10-13 = s2

The molar solubility of MY in water is less than of NY3
Question 17.
What is the pH of the resulting solution when equal volumes of 0.1M NaOH and 0.01M HCl are mixed?
(a) 2.0
(b) 3
(c) 7.0
(d) 12.65
Answer:
(d) 12.65
x ml of 0.1 m NaOH + x ml of 0.01 M HCI
No. of moles of NaOH = 0.1 x x x 10-3 = 0.l x x 10-3
No. of moles of HCl = 0.01 x x x 10-3 = 0.01 x x 10-3
No. of moles of NaOH after mixing = 0.1x x 10-3 – 0.01x x 10-3
= 0.09x x 10-3
Concentration of NaOH =
![]()
[OH–] = 0.045
pOH = – log (4.5 x 10-2)
= 2 – log 4.5
= 2 – 0.65 = 1.35
pH = 14 – 1.35 = 12.65
Question 18.
The dissociation constant of a weak acid is 1 x 10-3 . In order to prepare a buffer solution with a pH =4, the [Acid] / [Salt] ratio should be ………………..
(a) 4:3
(b) 3:4
(c) 10:1
(d) 1:10
Answer:
(d) 1:10
Ka = 1 x 10-3 ; pH = 4

Question 19.
The pH of 10-5 M KOH solution will be …………..
(a) 9
(b) 5
(c)19
(d) none of these
Answer:
(a) 9
![]()
[OH–] = 10-5M.
pH = 14 – pOH .
pH = 14 – ( – log [OH–])
= 14 + log [OH–] = 14 + log 10-5
= 14 – 5 = 9
Question 20.
H2PO4– the conjugate base of …………….
(a) PO4
(b) P2O5
(c) H3PO4
(d) HPO42-
Answer:
(c) H3PO4
![]()
H2PO4 is the conjugate base of H3PO4
Question 21.
Which of the following can act as lowery – Bronsted acid well as base?
(a) HCl
(b) SO42-
(c) HPO42-
(d) Br–
Answer:
(c) HPO42-
HPO42- can have the ability to accept a proton to form H2PO4.
It can also have the ability to donate a proton to form PO4-3.
Question 22.
The pH of an aqueous solution is Zero. The solution is ……………..
(a) slightly acidic
(b) strongly acidic
(c) neutral
(d) basic
Answer:
(b) strongly acidic
pH = – log10[H+]
[H+] =10-pH
= 100 = 1
[H+] = 1 M
The, solution is strongly acidic
Question 23.
The hydrogen ion concentration of a buffer solution consisting of a weak acid and its salts is given by ………………
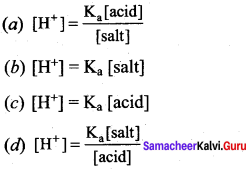
Answer:
According to Henderson equation
![]()
According to Henderson equation

Question 24.
Which of the following relation is correct for degree of hydrolysis of ammonium acetate?

Answer:
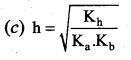
Question 25.
Dissociation constant of NH4OH is 1.8 x 10-5 the hydrolysis constant of NH4Cl would be …………….
(a) 1.8 x 10-19
(b) 5.55 x 10-10
(c) 5.55 x 10-5
(d) 1.80 x 10-5
Answer:
(b) 5.55 x 1010
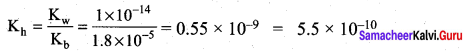
II. Answer the following questions.
Question 1.
What are lewis acids and bases? Give two example for each.
Answer:
1. Lewis acids:
- Lewis acid is a species that accepts an electron pair.
- Lewis acid is a positive ion (or) an electron deficient molecule.
- Example, Fe2+, CO2, BF3, SiF4 etc…
2. Lewis bases:
- Lewis base is a species that donates an electron pair.
- Lewis base is an anion (or) neutral molecule with atleast one lone pair of electrons.
- Example, NH3, F–, CH2 = CH2, CaO etc….
Question 2.
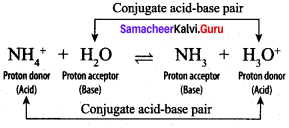
Discuss the Lowry – Bronsted concept of acids and bases.
Answer:
According to Lowry – Bronsted concept, an acid is defined as a substance that has a tendency to donate a proton to another substance and base is a substance that has a tendency to accept a proton from other substance. When hydrogen chloride is dissolved in water, it donates a proton to the later. Thus, HCl behaves as an acid and H2O is base. The proton transfer from the acid to base can be represented as
HCl + H2O \(\rightleftharpoons\) H3O+ + Cl–
When ammonia is dissolved in water, it accepts a proton from water. In. this case, ammonia (NH3) acts as a base and H2O is acid. The reaction is represented as
H2O + NH3 \(\rightleftharpoons\) NH4+ + OH–
Let us consider the reverse reaction in the following equilibrium
![]()
H3O+ donates a proton to Cl– to form HCI i.e., the products also behave as acid and base. In general, Lowry – Bronsted (acid – base) reaction is represented as
Acid1 + Base2 \(\rightleftharpoons\) Acid2 + Base1
The species that remains after the donation of a proton is a base (Base1)and is called the conjugate base of the Bronsted acid (Acid1). In other words, chemical species that differ only by a proton are called conjugate acid – base pairs. Conjugate acid – base pair
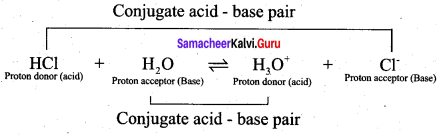
HCl and Cl–, H2O and H3O are two conjugate acid – base pairs. i.e., Cl– is the conjugate base of the acid HCl (or) HCl is conjugate acid of Cl– Similarly H3O is the conjugate acid of H2O. Limitations of Lowry – Bronsted theory. Substances like BF3 , AICl3 etc., that do not donate protons are known to behave as acids.
Question 3.
Indentify the conjugate acid base pair for the following reaction in aqueous solution.
- HS– (aq) + HF \(\rightleftharpoons\) F– (aq) + H2S (aq)
- HPO42- + SO32- \(\rightleftharpoons\) PO43- + HSO3–
- NH4+ + CO32- \(\rightleftharpoons\) NH3 + HCO3–
Answer:
1.

• HF and F– , HS– and H2S are two conjugate acid – base pairs.
• F– is the conjugate base of the acid HF (or) HF is the conjugate acid of F–
• H2S is the conjugate acid of HS– (or) HS– is the conjugate base of H2S.
2.
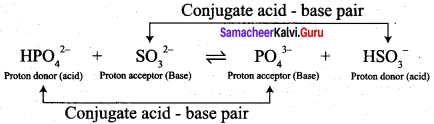
• HPO42- and PO43-, SO32- and HSO3– are two conjugate acid – base pairs.
.PO43- is the conjugate base of the acid HPO42- (or) HPO42- is the conjugate acid of PO4.
•HSO3– is the conjugate acid of SO32- (or) SO32- is the conjugate base of HSO3–.
3.

• NH+ and NH3, CO32- and HCO3– are two conjugate acid – base pairs.
• HCO3– is the conjugate of acid CO32- (or) CO32- is the conjugate bases of HCO3–.
• NH3 is the conjugate base of NH4+ (or) NH4+ is the conjugate acid of NH3.
Question 4.
Account for the acidic nature of HCIO4. In terms of Bronsted – Lowry theory, identify its conjugate base.
Answer:
HClO4 \(\rightleftharpoons\) H+ + ClO4–
1. According to Lowry – Bronsted concept, a strong acid has weak conjugate base and a weak acid has a strong conjugate base.
2. Let us consider the stabilities of the conjugate bases ClO4– , ClO3–, CIO2– and ClO– formed from these acid HClO4, HClO3, HCIO2, HOCI respectively.
These anions are stabilized to greater extent, it has lesser attraction for proton and therefore, will behave as weak base. Consequently the corresponding acid will be strongest because weak conjugate base has strong acid and strong conjugate base has weak acid.
3. The charge stabilization mercases in the order, ClO– < ClO2– < ClO3– < ClO4– .
This means ClO4– will have maximum stability and therefore will have minimum attraction for W. Thus CIO4– will be weakest base and its conjugate acid HCIO4 is the strongest acid.
4. CIO4– is the conjugate base of the acid HClO4.
Question 5.
When aqueous ammonia is added to CuSO4 solution, the solution turns deep blue due to the formation of tetrammine copper (II) complex, [Cu(H2O)6]2+(aq) + 4NH3 (aq) \(\rightleftharpoons\) [Cu(NH3)4]2+ (aq), among H2O and NH3 which is stronger Lewis base.
Answer:
Copper (II) sulphate solution, for example contains the blue hexaaqua copper (II) complex ion. In the first stage of the reaction, the ammonia acts as a Bronsted – Lowry base. With a small amount of ammonia solution, hydrogen ions are pulled off two water molecules in the hexaaqua ion. This produces a neutral complex, one carrying no charge.
If you remove two positively charged hydrogen ions from a 2+ ion, then obviously there isn’t going to be any charge left on the ion. Because of the lack of charge, the neutral complex isn’t soluble in water and so you get a pale blue precipitate. [Cu(H2O)6]2+ + 2NH3 [Cu(H2O)4OH] + 2NH4+
This precipitate is often written as Cu(OH)2 and called copper (II) hydroxide. The reaction is reversible because ammonia is only a weak base. That precipitate dissolves if you add an excess of ammonia solution, giving a deep blue solution. The ammonia replaces four of the water molecules around the copper to give tetramminc diaqua copper (II) ions. The ammonia
uses its lone pair to form a coordinate covalent bond with the copper. It is acting as an electron pair donor – a Lewis base.

Question 6.
The concentration of hydroxide ion in a water sample is found to be 2.5 x 10-6 M. Identify the nature of the solution.
Answer:
The concentration of OH ion in a water sample is found to be 2.5 x 10 M
pOH = – log10 [OH– ]
pOH = – 1og10 [2.5 x 10-6]
= – log10 [2.5] – log10 [10-6]
= – 0.3979 – ( – 6)
= – 0.3979 + 6
pOH = 5.6
We know that,
pH + pOH = 14
pH + 5.6 = 14
pH = 14 – 5.6
pH = 8.4
pH = 8.4, shows the nature of the solution is basic.
Question 7.
A lab assistant prepared a solution by adding a calculated quantity of HCl gas 25°C to get a solution with [H3O+] = 4 x 105 M. Is the solution neutral (or) acidic (or) basic.
Answer:
[H3O+] = 4 x M
pH = – log10 [H3O+]
pH = – 1og10[4 x 105]
pH = – log10 [4] – log10 [10-5]
pH = – 0.6020 – ( – 5) = – 0.6020 + 5
pH = 4.398
Therefore, the solution is acidic.
Question 8.
Calculate the pH of 0.04 M HNO3 Solution.
Answer:
Concentration of HNO3 = 0.04M
[H3O+] = 0.04 mol dm-3
pH = – 1og[H3O+]
= – log (0.04)
= – log(4 x 10-2)
= 2 – log4 = 2 – 0.6021
= 1.3979 = 1.40
Question 9.
Define solubility product.
Answer:
Solubility product:
It is defined as the product of the molar concentration of the constituent ions, each raised to the power of its stoichiometric coefficient in a balanced equilibrium equation.
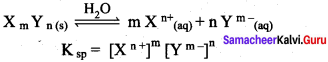
Question 10.
Define ionic product of water. Give its value at room temperature.
Answer:
1. The product of concentration of H+ and OH– ions in water at a particular temperature is known as ionic product.
2. The ionic product of water at room temperature (25°C) is,
Kw = [H+] [OH+] (or)
Kw= [H3O+] [OH+]
Kw =(1 x 10-7) (1 x 10-7)
Kw= 1 x 10-14 mol2 dm-6
Question 11.
Explain common ion effect with an example.
Answer:
Common ion Effect:
When a salt of a weak acid is added to the acid itself, the dissociation of the weak acid is suppressed further. Acetic acid is a weak acid. It is not completely dissociated in aqueous solution and hence the following equilibrium exists.
CH3COOH (aq) \(\rightleftharpoons\) H+(aq)+ CH3COO– (aq)
However, the added salt, sodium acetate, completely dissociates to produce Na+ and CH3COO ion.
CH3COONa (aq) → Na+ (aq) + CH3COO (aq) Hence, the overall concentration ofCH3COO is increased, and the acid dissociation equilibrium is disturbed.
We know from Le chatelier’s priñciple that when a stress is applied to a system at equilibrium, the system adjusts itself to nullify the effect produced by that stress. So, in order to maintain the equilibrium, the excess CH3COO– ions combines with H ions to produce much more unionized CH3COOH i.e.,
the equilibrium will shift towards the left. In other words, the dissociation of CH3COOH is suppressed. Thus, the dissociation of a weak acid (CH3COOH) is suppressed in the presence of a salt (CH3COONa) containing an ion common to the weak electrolyte. It is called the common ion effect.
Question 12.
Derive an expression for Ostwald’s dilution law.
Answer:
Ostwald’s dflution law:
It relates the dissociation constant of the weak acid (Ka) with its degree of dissociation (α) and the concentration (c). Considering a weak acid, acetic acid. The dissociation of acetic acid can be represented as,
CH3COOH \(\rightleftharpoons\) CH3COO– + H+
The dissociation constant of acetic acid is,
![]()

Substituting the equilibrium concentration in equation

We know that weak acid dissociates only to a very small extent compared to one, a is so small.
equation (1) becomes,

Similarly, for a weak base,
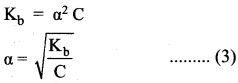
The concentration of H can be calculated using the Ka value as below,

Substituting a value in equation (2),
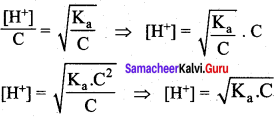
For weak base
![]()
Question 13.
Define pH.
Answer:
pH of a solution is defined as the negative logarithm of base 10 of the molar concentration of the hydronium ions present in the solution.
pH = – log10 [H3O] (or) pH = – log10 [H+]
Question 14.
Calculate the pH of 1.5 x 10-3 M solution of Ba(OH)2
Answer:
![]()
[OH–] = 3 x 103M.
[pH + pOH = 14]
pH = 14 – pOH
pH = 14 – ( – log [OH–])
= 14 + log [OH–]
= 14 + log (3 x 10-3)
= 14 + log 3 + log 10-3
= 11 + 0.4771
pH = 11.48
Question 15.
50 ml of 0.05 M HNO3 is added to 50 ml of 0.025 M KOH. Calculate the pH of the resultant solution.
Solution.
Number of moles of HNO3 = 0.05 x 50 x = 2.5 x 10-3
Number of moles of KOH = 0.025 x 50 x 10-3 = 1.25 x 10-3
Number of moles of HNO3 after mixing = 2.5 x 10-3 – 1.5 x 10-3
= 1.25 x 10-3
![]()
After mixing, total volume = 100 ml = 100 x 10-3 L
![]()
pH = – log [H+]
pH = – log (1.25 x 10-2) = 2 – 0.0969
= 1.9031
Question 16.
The Ka value for HCN is 10-9. What is the pH of 0.4 M HCN solution?
Answer:
Ka =10-9
c = O.4M
pH = – log [H+]
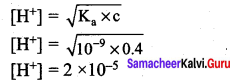
∴ pH = – log(2 x 10-5)
= – log 2 – log (10-5)
= – 0.3010 + 5
pH = 4.699
Question17.
Calculate the extent of hydrolysis and the pH of 0.1 M ammonium acetate Given that.
Ka = Kb = 1.8 x 10-5
Solution.
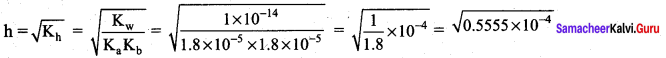
= 0.7453 x 10-2
pH = \(\frac { 1 }{ 2 }\) pKw + \(\frac { 1 }{ 2 }\) pKa – \(\frac { 1 }{ 2 }\) pKb
Given that Ka = Kb = 1.8 x 10-5
if Ka = Kb, then. pKa = pKb
pH = \(\frac { 1 }{ 2 }\) pKw = \(\frac { 1 }{ 2 }\) (14) = 7
Question 18.
Derive an expression for the hydrolysis constant and degree of hydrolysis of salt of strong acid and weak base.
Answer:
Let us consider the reactions between a strong acid, HCl, and a weak base, NH4OH, to produce a salt, NH4Cl, and water.
HCl (aq) + NH4OH (aq) \(\rightleftharpoons\) NH4Cl (aq) + H2O (I)
NH4CI(aq) → NH4+ + Cl– (aq)
NH4+ is a strong conjugate acid of the weak base NH4OH and it has a tendency to react with OH– from water to produce unionised NH4OH shown below.
NH4+ (aq) + H2O (1) \(\rightleftharpoons\) NH4OH (aq) + H+(aq)
There is no such tendency shown by Ct and therefore [H+] > [OH–] the solution is acidic and the pH is less than 7.
As discussed in the salt hydrolysis of strong base and weak acid. In this case also, we can establish a relationship between the Ka and Kb as
Kh.Kb = Kw
Let us calculate the Kb value in terms of degree of hydrolysis (h) and the concentration of salt
Kh = h2 C and
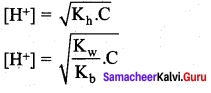
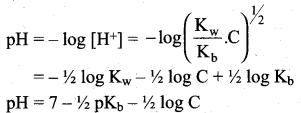
Question 19.
Solubility product of Ag2CrO4 is 1 x 10-12. What is the solubility of Ag2CrO4 in 0.01 M AgNO3 solution?
Answer:
Solubility product of Ag2CrO4,
Ksp = 1 x 10-2
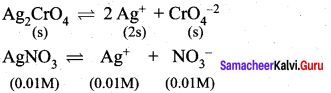
Ksp = [Ag+]2 [ CrO42-]
[Ag+] = 2s +0.01
0.01 >> 2s
[Ag+] = 0.01M
[CrO4-2] = s
Ksp = (0.01)2. (s)
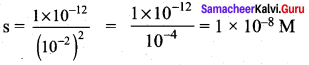
Question 20.
Write the expression for the solubility product of Ca3(PO4)2
Answer:
Ca3(PO4)2 (s) \(\rightleftharpoons\) 3Ca2+ (3s) + 2PO43- (2s)
Solubility of Ca3(PO4)2 is,
Ksp = [Ca2+]3 . [PO43-]2
Ksp = (3s)3 . (2s)2
Ksp= (27 s3) . (4s2)
Ksp = 108s5.
Question 21.
A saturated solution, prepared by dissolving CaF2(s) in water, has [Ca2+] = 3.3 x 10-4 M. What is the Kspof CaF2?
Answer:
CaF2 (s) \(\rightleftharpoons\) Ca2+(aq) + 2F–(aq)
[F–] = 2 [Ca2+] = 2 x 33 x 10-4 M
= 6.6 x 10-4 M
= [Ca2+] [F–]2
= (3.3 x 10-4) (6.6 x 10-4)2
= 1.44 x 10-10
Question 22.
Ksp of AgCI is 1.8 x 10-10. Calculate molar solubility in 1 M AgNO3
Answer:
AgCI(s) Ag+(aq) + Cl–(aq)
x = solubility of AgCI in 1M AgNO3
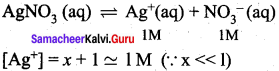
[Cl–] = x
Ksp = [Ag+] [Cl–]
1.8 x 10-10 = (1) (x)
x = 1.8 x 10-10M
Question 23.
A particular saturated solution of silver chromate Ag2CrO4 has [Ag+] = 5 x 10-5 and [CrO4]2- = 4.4 x 10 M. What is the value of for Ag2CrO4?
Answer:
Ag2CrO4 (s) \(\rightleftharpoons\) 2Ag+(aq) + CrO42-(aq)
Ksp = [Ag+]2 [CrO42-]
= (5 x 10-5)2 (4.4 x 10-4)
= 1.1 x 10-12
Question 24.
Write the expression for the solubility product of Hg2CI2.
Answer:
![]()
Question 25.
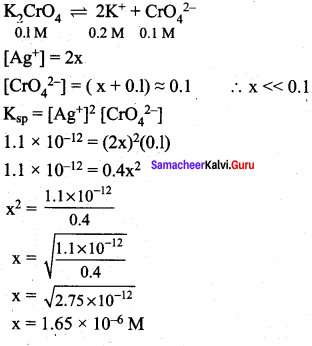
Ksp of Ag2CrO4 is 1.1 x 10-12 What is solubility of Ag2CrO4 in 0.1M K2CrO4.
Answer:
![]()
x is the solubility of Ag2CrO4 in 0.1 M K2CrO4
Question 26.
Will a precipitate be formed when 0.150 L of 0.1 M Pb(NO3)2 and 0.100 L of 0.2 M NaCl are mixed? (PbCI2) = 1.2 x 10-5.
Answer:
When two or more solution are mixed, the resulting concentrations are differnet from the original.
Total volume 0.250L
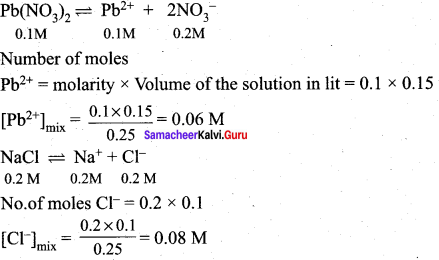
Precipitation of PbCI2 (s) occurs if [Pb2+][Cl–]2 > Ksp
[Pb2+][Cl–]2 = (0.06)(0.08)2
= 3.84 x 10-4
Since ionic product [Pb2+][Cl–]2 > Ksp PbCl2 is precipitated.
Question 27.
of Al(OH)3 is 1 x 10-15 M. At what pH does 1.0 x 10-13 M AI3+ precipitate on the addition of buffer of NH4CI and NH4OH solution?
Answer:
Al(OH)3 Al3+ (aq) + 3OH– (aq)
Ksp = [Al3+] [OH–]3
Al(OH)3 precipitates when
[Al3+] [OH–]3 > Ksp
(1 x 10-3)[OH–]3 > Ksp
[OH–]3 > 1 x 10-12
[OH–] > 1 x 10-4M
[OH–] = l x 10-4 M
pOH = – 1og10[OH–] = – log (1 x 10-4) = 4
pH = 14 – 4 = 10
Thus, Al (OH)3 precipitates at a pH of 10
Samacheer Kalvi 12th Chemistry Ionic Equilibrium Evaluate Yourself
Question 1.
Classify the following as acid (or) base using Arrhenius concept
- HNO3
- Ba(OH)2
- HlPO4
- CH3COOH
Answer:
1. HNO3:
Nitric acid, dissociates to give hydrogen ions in water.
HNO3 is acid.
2. Ba(OH)2:
Barium hydroxide, dissociates to give hydroxyl ions in water.
Ba(OH)2 is base.
3. H3PO4:
Orthophosphoric acid, dissociates to give hydrogen ions in water.
H3PO4 is acid.
4. CH3COOH:
Acetic acid, dissociates to give hydrogen ions in water.
CH3COOH is acid.
Question 2.
Write a balanced equation for the dissociation of the following in water and identify the conjugate acid base pairs.
- NH4
- H2SO4
- CH3COOH.
Answer:
1. NH4 + Conjugate acid-base pair
NH4+ and NH3, H2O and H3O+ are two conjugate acid – base pairs.
H2SO4 and CH3COO–, H2O and H3O+ are two conjugate acid-base pairs.
2. H2SO4:
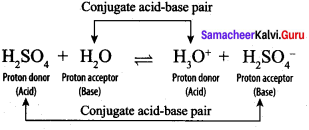
H2SO4 and HSO4–, H2O and H3O+ are two conjugate acid-base pairs.
3. CH3COOH:
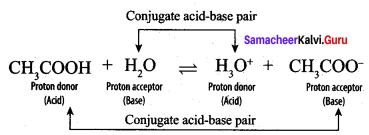
CH3COOH and CH3COO–, H2O and H3O+ are two conjugate acid-base pairs.
Question 3.
Identify the Lewis acid and the Lewis base in the following reactions.
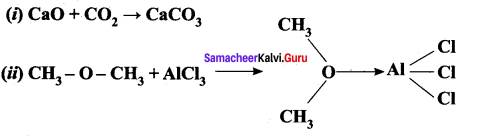
Answer:
(i). CaO + CO2 → CaCO3
- CaO – Lewis base – All metals oxides are Lewis bases
- CO2 – Lewis acid – CO2 contains a polar double bond.
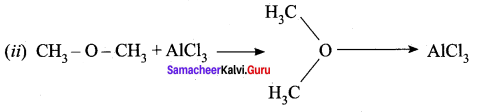
- CH3 – O – CH3 – Lewis base – Electron rich species
- AlCl3 – Lewis acid – AICI3 is electron deficient molecule.
Question 4.
H3BO3 accepts hydroxide ion from water as shown below
Answer:
H3BO3 (aq) + H2O(l) = B(OH)4– + H+
Predict the nature of H3BO3 using Lewis concept. Boric acid is also called as hydrogen borate or orthoboric acid. It is a weak mono basic Lewis acid of boron and it is written as B(OH)3. It accepts hydroxyl (OH–) ion from water. It does not dissociate to give hydronium (H3O+) ion rather forms metaborate ion and this ions in turn give H3O ion.
B(OH)3 + H2O [B(OH)4]– + H3O+ Hence it is considered as weak acid.
Question 5.
At a particular temperature, the Kw of a neutral solution was equal to 4 x 10-14. Calculate the concentration of [H3O+] and [OH–].
Answer:
Given solution is neutral
[H3O+] = [OH–]
Let [H3O+] = x ; then [OH–] = x
Kw = [H3O+] [OH–]
4 x 10-14 = x . x
x2 = 4 x 10-14
![]()
Question 6.
- Calculate pH of 10-8 M H2SO4
- Calculate the concentration of hydrogen ion in moles per litre of a solution whose pH is 5.4
- Calculate the pH of an aqueous solution obtained by mixing 50 ml of 0.2 M HCI with 50 ml 0.1 M NaOH
Answer:
1.

In this case the concentration of H2SO4 is very low and hence [H3O] from water cannot be neglected
[H3O+] = 2 x 10-8 (from H2SO4) + 10-7 (from water)
= 10-8(2+ 10)
= 12 x 10-8 = 1.2 x 10-7
pH = – log10[H3O+]
= – log10( 1.2 x 10-7)
= 7 – log101.2
= 7 – 0.0791 = 6.9209
2.
pH of the solution = 5.4
[H3O+] = antilog of (- pH)
= antilog of (- 5.4)
= antilog of (-6 + 0.6) = \(\overline{6} .6\)
= 3.981 x 10-6
i.e., 3.98 x 10-6 mol dm-3
3.
No. of moles of HCl = 0.2 x 50 x 10-3 = 10 x 10-3
No. of moles of NaOH =0.1 x 50 x 10-3 = 5 x 10-3
No. of moles of HCl after mixing = 10 x 10-3 – 5 x 10-3
= 5 x 10-3
after mixing total volume = 100 mL

[H3O+] = 5 x 10-2 M
pH = – log ( 5 x 10-2)
= 2 – log 5
= 2 – 0.6990
= 1.30
Question 7.
Kb for NH4OH is 1.8 x 10-5 Calculate the percentage of ionisation of 0.06 M ammonium hydroxide solution.
Answer:
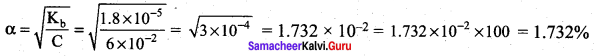
Question 8.
1. Explain the buffer action in a basic buffer containing equimolar ammonium hydroxide and ammonium chloride.
2. Calculate the pH of a buffer solution consisting of 0.4M CH3COOH and 0.4 M CH3COONa . What is the change in the pH after adding 0.01 mol of HCI to 500m1 of the above buffer solution.
Assume that the addition of HCI causes negligible change In the volume. Given: (K = 1.8 x 105).
Answer:
1. Dissociation of buffer components
NH4OH (aq) \(\rightleftharpoons\) NH4+ (aq) + OH– (aq)
NH4CI → NH4+ + Cl–
Addition of OH–
The added H+ ions are neutralized by NH4OH and there is no appreciable decrease in pH.
NH4OH(aq) + H+ \(\rightleftharpoons\) NH4+(aq) + H2O (1)
Addition of
NH4– (aq) + OH– (aq) → NH4OH (aq)
The added OH ions react with NH4 to produce unionized NH4OH . Since NH4OH is a weak base, there is no appreciable increase in pH.
2. pH of buffer
![]()
Addition of 0.01 mol HCI to 500 ml of buffer
Added [H+]
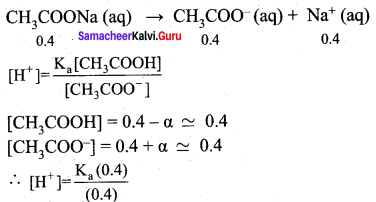
∴ pH = – log (1.8 x 10-5) = 4.74
Addition of 0.01 mol HCl to 500ml of buffer

Question 9.
1. How can you prepare a buffer solution of pH9. You are provided with 0.1 M NH4OH solution and ammonium chloride crystals. (Given: pKb for NH4OH is 4.7 at 25°C)
2. What volume of 0.6 M sodium formate solution is required to prepare a buffer solution of pH 4.0 by mixing it with 100 ml of 0.8 M formic acid. (Given: pKa for formic acid is 3.75.)
Answer:
1.
![]()
We know that pH + pOH = 14
9 + pOH = 14
= pOH = 14 – 9 = 5
![]()
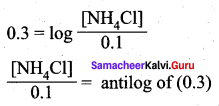
[NH4Cl] = 0.1 M x 1.995
= 0. 1995 M
=0.2 M
Amount of NH4CI required to prepare 1 litre 0.2 M solution = Strength of NH4CI x molar
mass of NH4CI
= 0.2 x 535
= 10.70 g
10.70 g ammonium chloride is dissolved in water and the solution is made up to one litre to get 0.2 M solution. On mixing equal volume of the given NH4OH solution and the prepared NH4CI solution will give a buffer solution with required pH value (pH = 9).
2.

[Sodium formate] = number of moles of HCOONa
= 0.6 x V x 10-3
[formic acid] = number of moles of HCOOH
= 0.8 x 100 x 10-3
[formic acid] = number of moles of HCOOH
= 0.8 x 100 x 10-3
= 80 x 10-3
4 = 3.75 + log \(\frac { 0.6V }{ 80 }\)
0.25 = log \(\frac { 0.6V }{ 80 }\)
antilog of 0.25 = \(\frac { 0.6V }{ 80 }\)
0.6V = 1.778 x 80
= 1.78 x 80
= 142.4
V = \(\frac { 142.4 mL }{ 0.6 }\) = 237.33 mL
Question 10.
Calculate the
- hydrolysis constant
- degree of hydrolysis and
- pH of 0.05M sodium carbonate solution pKa for HCO3– is 10.26.
Answer:
1. Hydrolysis constant
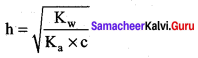
Given
Kw = 1 x 10-14
c = 0.05 M
PKa = 10.26
Ka = – log Ka
Ka = antilog of (- pKa)
Ka = antilog of (- 10.26)
Ka = 5.49 x 10-11
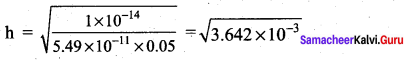
h = 6.034 x 10-2
2. Degree of hydrolysis
![]()
3.

Samacheer Kalvi 12th Chemistry Ionic Equilibrium Textbook Example problems solved
Question 1.
Identify the Lewis acid and the Lewis base in the following reactions.
Cr3+ + 6H2O → [Cr(H2O)6]3+
In the hydration of ion, each of six water molecules donates a pair of electron to Cr3+ to form the hydrated cation, hexaaquachromium (III) ion, thus, the lewis acid is Cr and the Lewis base H2O.
Question 2.
Calculate the concentration of OH– in a fruit juice which contains 2 x 10 M, H3O+ Ion. Identify the nature of the solution.
Answer:
Given that H3O+ = 2 x 10-3 M
Kw = [H3O+] [OH–]
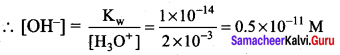
2 x 10-3 >> 0.5 x 10-11
i.e., [H3O+] >> [OH–], hence the juice is acidic in nature
Question 3.
Calculate the pH of 0.001M HCI solution
Answer:

H3O from the auto ionisation of H2O (10-7 M) is negligible when compared to the H3O from 10-3 M HCI.
Hence [H3O+] = 0.001 mol dm-3
pH = – log10 [H3O+]
= – log10 (0.001)
= – log10 (10-3) = 3
Question 4.
Calculate pH of 10-7 M HCI
Answer:
If we do not consider [H3O+] from the ionisation of H2O, then [H3O+] = [HCl] = 107M i.e., pH =7, which is a pH of a neutral solution. We know that HCI solution is acidic whatever may be the concentration of HCI i.e, the pH value should be less than 7. In this case the concentration of the acid is very low (10-7M). Hence, the H3O+ (10-7M) formed due to the auto ionisation of water cannot be neglected. So, in this case we should consider [H3O+] from ionisation of H2O
Answer:
[H3O+] = 10-7 (from HCl) + 10-7(from water)
= 10-7 (1+1)
= 2 x 10
pH = – log10 [H3O]
= – log10(2 x 107) = – [log2 + log 10-7]
= – log 2 – ( – 7). 1og10
= 7 – log 2
= 7 – 0.3010 = 6.6990
= 6.70
Question 5.
A solution of 0.10 M of a weak electrolyte is found to be dissociated to the extent of 1.20% at 25°C. Find the dissociation constant of the acid.
Answer:
Given that α = 1.20% = \(\frac { 1.20 }{ 100 }\) = 1.2 x 10-2
Ka = α2c = (1.2 X 10-2)2 (0.1)
= 1.44 x 10-4 x 10-1 = 1.44 x 10-5
Question 6.
Calculate the pH of 0.1M CH3COOH solution. Dissociation constant of acetic acid is 1.8 x 10-5.
Answer:
pH = – log[H+]
For weak acids,

Question 7.
Find the pH of a buffer solution containing 0.20 mole per litre sodium acetate and 0.18 mole per litre acetic acid. Ka for acetic acid is 1.8 x 10-5
![]()
Given that Ka = 1.8 x 10-5
pKa = – log (1.8 x 10-5)
= 5 – log 1.8
= 5 – 0.26 = 4.74
pH = 4.74 + log \(\frac { 0.20 }{ 0.18 }\)
= 4.74 + log \(\frac { 10 }{ 9 }\)
= 4.74 + log 10 – log 9
= 4.74 + 1 – 0.95
= 5.74 – 0.95
= 4.79
Question 8.
What is the pH of an aqueous solution obtained by mixing 6 gram of acetic acid and 8.2 gram of sodium acetate and making the volume equal to 500 ml. (Given: K for acetic acid is 8 x 10)
Answer:
According to Henderson – Hessalbalch equation,
![]()
Given that Ka = 1.8 x 10-5
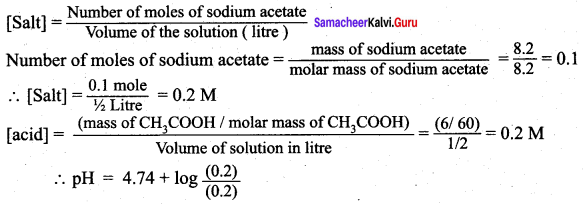
pH = 4.74 + log 1
pH = 4.74 + 0 = 4.74
Question 9.
Calculate
- the hydrolysis constant,
- degree of hydrolysis and
- pH of O.1 M CH3COONa solution (pKa for CH3COOH is 4.74).
Answer:
CH3COONa is a salt of weak acid (CH3COOH) and a strong base (NaOH). Hence, the solutions is alkaline due to hydrolysis.
CH3COO (aq) + H2O (aq) \(\rightleftharpoons\) CH3COOH (aq) + OH– (aq)
Give that pKa = 4.74
pKa = – log Ka
i.e., Ka = antilog of ( – PKa)
= antilog of ( – 4.74)
= antilog of( – 5 + 0.26)
10-5 x 1.8


Question 10.
Establish a relationship between the solubility product and molar solubility for the following
- BaSO4
- Ag2(CrO4)
Answer:
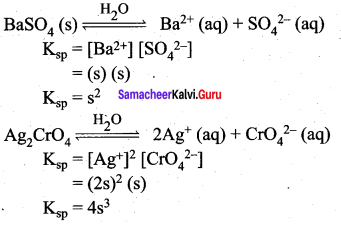
Samacheer Kalvi 12th Chemistry Ionic Equilibrium Multiple Additional Questions
Samacheer Kalvi 12th Chemistry Ionic Equilibrium 1 Marks Questions and Answers
I. Choose the best answer.
Question 1.
Which one of the following buffer is present in blood?
(a) CH3COOH + CH3COONa
(b) NH4OH + NH4Cl
(c) H2CO3 + NaHCO3
(d) HCI + NaCl
Answer:
(c) H2CO3 + NaHCO3
Question 2.
Which of the following is mostly used in fertilizer industry?
(a) Lactic acid
(b) Sulphuric acid
(c) Tannic acid
(d) Carbonic acid
Answer:
(b) Sulphuric acid
Question 3.
Which of the following is present in an antacid tablet?
(a) NaOH
(b) Mg(OH)2
(c) Al(OH)3
(d) either (b) or (c)
Answer:
(d) either (b) or (c)
Question 4.
The acid present in milk is …………..
(a) Lactic acid
(b) Tannic acid
(c) Tartaric acid
(a) Acetic acid
Answer:
(a) Lactic acid
Question 5.
Consider the following statements.
(i) Acid tastes sour
(ii) Acid turns red litmus to blue
(iii) Acid reacts with metals and liberates hydrogen gas
Which of the above statement is I are correct?
(a) (i) only
(b) (i) & (ii)
(c) (i) & (iii)
(d) (ii) only
Answer:
(c) (i) & (iii)
Question 6.
Consider the following statements.
(i) Acid tastes sour.
(ii) Acid turns blue litmus to red
(iii) Acid has a tendency to accept a proton from other substances.
Which of the above statement is I are not correct?
(a) (i) & (ii)
(b) (ii) & (iii)
(c) (iii) only
(a) (ii) only
Answer:
(c) (iii) only
Question 7.
Which of the following can act as an acid as well as base by Lowry – Bronsted theory?
(a) H2O
(b) NH3
(c) NH4OH
(d) Ca(OH)2
Answer:
(a) H2O
Question 8.
In the reaction HCI + H2O \(\rightleftharpoons\) H3O + Cl– which one of the acid-base pair?
(a) HCl + H3O+
(b) HCI + CI–
(c) H3O + Cl
(d) H2O + Cl–
Answer:
(b) HCI + CI–
Question 9.
Which of the following is considered as Lewis acid?
(a) NH3
(b) BF3
(c) HF
(d) HCl
Answer:
(b) BF3
Question 10.
Which of the following is considered as Lewis base?
(a) BF3
(b) AICI3
(c) HCI
(d) NH3
Answer:
(d) NH3
Question 11.
Consider the following statements.
(i) A Lewis acid is a species that accepts an electron pair.
(ii) A Lewis acid is a species that donates an electron pair.
(iii) The ligand act as Lewis base.
Which of the above statement is / are not correct?
(a) (i) only
(b) (ii) only
(c) (I) & (ii)
(d) (ii) & (iii)
Answer:
(b) (ii) only
Question 12.
In [Cr(H2O)6]3+ which one of the following acts as Lewis acid?
(a) Cr
(b) Cr3+
(c) (HO)6
(d) Cr3-
Answer:
(b) Cr3+
Question 13.
In [Cr(H2O)6]3+ which one of the following acts as Lewis base?
(a) H2O
(b) H3O+
(c) Cr3+
(d) Cr
Answer:
(a) H2O
Question 14.
Among the following which is the strongest acid?
(a) Formic acid
(b) Acetic acid
(c) Hydrochloric acid
(d) Lactic acid
Answer:
(c) Hydrochloric acid
Question 15.
Which of the following is the weak acid?
(a) HCl
(b) H2SO4
(c) HNO3
(d) CH3COOH
Answer:
(d) CH3COOH
Question 16.
Identify the weakest acid?
(a) H3O+
(b) H2 SO4
(c) OH–
(d) CH3COOH
Answer:
(c) OH–
Question 17.
Which one of the following is the very weak base?
(a) NO2–
(b) NO3–
(c) NH2–
(d) O2-
Answer:
(b) NO3–
Question 18.
Which one of the following is the strong base?
(a) ClO4–
(b) HSO4
(c) O2-
(d) F–
Answer:
(c) O2-
Question 19.
Which of the following is the weak base?
(a) H–
(b) OH–
(c) HSO4
(d) F–
Answer:
(d) F–
Question 20.
The value of ionic product of water at 25°C is ………………..
(a) 1 x 10-7
(b) 1 x 107
(c) 1 x 10-14
(d) 1 x 1014
Answer:
(c) 1 x 10-14
Question 21.
Consider the following statements.
(i) The dissociation of water is an exothermic reaction.
(ii) With the increase in temperature, the ionic product of water value decreases.
(iii) With the increase in temperature, the ionic product of water value increases.
Which of the above statement is / are correct?
(a) (i) and (ii)
(b) (ii) only
(c) (iii) only
(d) (ii) & (iii)
Question 22.
Which of the following is a neutral solution?
(a) Aqueous NaCl solution
(b) Aqueous NaOH solution
(c) Aqueous HCl solution
(d) Aqueous NH3
Answer:
(a) Aqueous NaCl solution
Question 23.
The pH of a neutral solution is ………..
(a) less than 7
(b) more than 7
(c) equal to 7
(d) 14
Answer:
(a) less than 7
Question 24.
If the pH of a solution is less than 7, it is called ………………… solution.
(a) Basic
(b) Acidic
(c) Neutral
(d) Amphoteric
Answer:
(b) Acidic
Question 25.
If the pH of a solution is more than 7, it is called ……………. solution.
(a) Basic
(b) Acidic
(c) Neutral
(d) Amphoteric
Answer:
(a) Basic
Question 26.
The pH value of water is …………..
(a) 14
(b) 7
(c) 3
(d) 1
Answer:
(b) 7
Question 27.
The pH of drain cleaner is ………..
(a) 7
(b) 1
(c) 14
(d) 0
Answer:
(c) 14
Question 28.
The pH of the battery acid is ………….
(a) 7
(b) 1
(c) 14
(d) 0
Answer:
(d) 0
Question 29.
The pH of 0.001 M HCI solution is ………
(a) 3
(b) 2
(c) 1
(d) 11
Answer:
(a) 3
Solution:
[H3O+] = 0.001 mol dm-3
pH = – log10 [H3O+] = – log10 [0.001]
= – 1og10 [10-3] = 3
pH = 3
Question 30.
The pH of 0.01 M HCl solution is …………..
(a) 3
(b) 2
(c) 1
(d) 10
Answer:
(b) 2
Solution:
[H3O+] = 0.01 M
pH = – 1og10 [H3O+] = – log10 [0.01]
= – 1og10[10-3] = 3
pH = 3
Question 31.
What is the pH of 0.1 M HCI solution?
(a) 1
(b) 2
(c) 13
(d) 3
Answer:
(a) 1
Solution:
[H3O+] = 0.1 M
pH = – log10[H3O+] = – log10[0.1]
= – log10[10-1]
pH = l
Question 32.
Consider the following statements.
(i) Degree of dissociation (a) is the fraction of the total number of moles of a substance that dissociates at equilibrium.
(ii) When the dilution increases by 100 times, the dissociation increases by 100 times.
(iii) When the dilution increases by 100 times, the dissociation increases by 10 times.
Which of the above statement is I are correct?
(a) (ii) only
(b) (i) & (iii)
(c) (iii) only
(d) (I) only
Answer:
(a) (ii) only
Question 33.
Which of the following is not a buffer solution?
(a) CH3COOH + CH3COONa
(b) NH4OH + NH4Cl
(c) H2CO3 + NaHCO3
(d) NaOH + NaCI
Answer:
(d) NaOH + NaCI
Question 34.
The mathematical expression of buffer capacity is …………
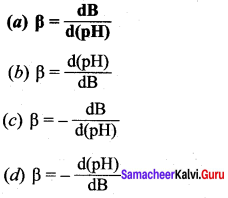
Answer:
![]()
Question 35.
Which one of the following is not correct?
(a) pH+pOH = 14
(b) pH = 7 +\(\frac { 1 }{ 2 }\) pKa – \(\frac { 1 }{ 2 }\) pKb
(c) pH x pOH = 1 x 1014
(d) pH = – log10 [H3O+]
Answer:
(c) pH x pOH = 1 x 1014
Question 36.
The chemical present in kidney as stones is …………..
(a) CaCl2
(b) Ca(CO3)2
(c) Calcium nitrate
(d) Calcium oxalate
Answer:
(d) Calcium oxalate
Question 37.
If the pH of an aqueous solution is 7, the solution is …………..
(a) slightly acidic
(b) strongly acidic
(c) neutral
(d) basic
Answer:
(c) neutral
Question 38.
Cl– is the conjugate base of …………..
(a) HClO4
(b) HCI
(c) ClO4–
(d) HClO3
Answer:
(b) HCI
Question 39.
The conjugate base of H2O and H2SO4 are …………..
(a) OH– and HSO4
(b) H4O and SO42-
(c) OH and SO42-
(d) H3O and HSO4
Answer:
(a) OH– and HSO4
Question 40.
The Ksp of AgI is 1.5 x 10-16. On mixing equal volume of the following solutions, precipitation will occur only with ………….
(a) 10-7MAg+ and 10-19M I–
(b) 10-8MAg+ and 10-8M I–
(c) 10-16 M Ag+ and 10-16 M I–
(d) 10-9 M Ag+ and 10-9 M I–
Answer:
(b) 10-8MAg+ and 10-8M I–
Ksp of AgI = 1.5 x 10-16
10-8 M Ag+ and 10-8 M I
Ionic product = 10-16 = Ksp
Question 41.
The strongest Bronsted base in the following anion is ……………
(a) ClO–
(b) ClO2-
(c) ClO3-
(d) ClO4-
Answer:
(a) ClO–
Solution:
HClO is the weakest acid and its conjugate base ClO– is the strongest base.
Question 42.
Calculate the hydrolysis constant of the salt containing NO2.
Given the Ka for HNO2 = 4.5 x 10-10
(a) 2.22 x 10-5
(b) 2.02 x 105
(c) 4.33 x 104
(d) 3.03 x 10-5
Answer:
(a) 2.22 x 10-5
Solution:
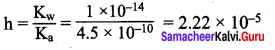
Question 43.
Electrophiles are usually ……….
(a) Lewis acid
(b) Lewis base
(c) Bronsted acid
(d) Bronted base
Answer:
(a) Lewis acid
Solution:
Lewis acid are electrophile because they accept electron pair.
Question 44.
Which one is a Lewis acid?
(a) CIF3
(b) H2O
(c) NH3
(d) OH
Answer:
(a) CIF3
Solution:
CIF3 have vacant d-orbital in central atom.
Question 45.
An aqueous solution of ammonium acetate is …………….
(a) faintly acidic
(b) faintly basic
(c) fairly acidic
(d) Almost neutral
Answer:
(d) Almost neutral
Solution:
It is a salt of weak acid and weak base.
Question 46.
The dissociation constant of a weak acid is 1.0 x 10-10. The equilibrium constant for the reaction with strong base is …………
(a) 1.0 x 10-5
(b) 1.0 x 10-9
(c) 1.0 x 109
(d) 1.0 x 1014
Answer:
(c) 1.0 x 109
Solution:

Question 47.
Arrange the acids
(i) H2SO3
(ii) H3PO3 and
(iii) HClO3in the decreasing order of acidity.
(a) (i) > (iii) > (ii)
(b) (i) > (ii) > (iii)
(c) (ii) > (iii) > (I)
(d) (iii) > (i)> (ii)
Answer:
(d) (iii) > (i)> (ii)
Solution:
Acidity is directly proportional to oxidation number. As the oxidation number of S, P and Cl in H2SO3, H3PO3 and HCIO3 is +4, +3, +5 respectively. So decreasing order of acidity will
be (iii) > (I) > (ii)
Question 48.
The pH of 0.1 M solution of a weak monoprotic acid 1% ionised is ………..
(a) 1
(b) 2
(c) 3
(d) 4
Answer:
(c) 3
Solution:
Conc = 0.1 M
α = 1
= 0.1 x \(\frac { 1 }{ 100 }\) = 10-3
[H+] = 10-3
∴ pH = 3
Question 49.
Ksp for Cr(OH)3 is 2.7 x 10-3. What is the solubility in moles / litre?
(a) 1 x 10-8
(b) 8 x 10-8
(c) 1.1 x 10-8
(d) 0.18 x 10-8
Answer:
(b) 8 x 10-8
Solution:
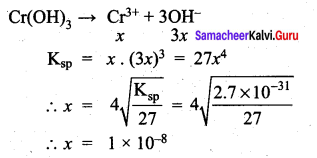
Question 50.
pKa of acetic acid is 4.74. The concenttation of CH3COONa is 0.01 M. The pH of CH3COONa
is …………..
(a) 3.37
(b) 4.37
(c) 4.74
(d) 0.474
Answer:
(a) 3.37
Solultion:
[H+] = c.α = \(\sqrt{\mathrm{K}_{\mathrm{a}} \cdot \mathrm{C}}\)
pH = – log \(\left(\mathrm{K}_{\mathrm{a}} \cdot \mathrm{C}\right)^{1 / 2}\)
= \(\frac { 1 }{ 2 }\) [-1ogKa – log c]
= \(\frac { 1 }{ 2 }\) [4.74 – log 10-2]
= \(\frac { 1 }{ 2 }\) [4.74 + 2] = 3.37
pH = 3.37
Question 51.
One litre of water contains 10 mol hydrogen ions. The degree of ionisation in water will be …………….
(a) 8 x 10-7
(b) 0.8 x 10-9
(c) 3.6 x 10-7
(d) 3.6 x 10-9
Answer:
(a) 8 x 10-7
Solution:
1 litre of water contains 1000/18 mole.
So, degree of ionisation = \(\frac{10^{-7} \times 18}{1000}\) = 1.8 x 10-7
Question 52.
If the solubility product of lead iodide (PbI2) is 3.2 x 10-8. Then its solubility in moles / litre will be ………….
(a) 2 x 10-3.
(b) 4 x 10-4
(c) 1.6 x 10-5
(d) 1.8 x 10-5
Solution:
Ksp = 4s3
4s3 = 3.2 x 10-8
s = 2 x 10-3M
Question 53.
The pH of a soft drink is 3.82. Its hydrogen ion concentration will be ……………
(a) 1.96 x 10-2mol / L
(b) 1.96 x 1o-3 mol / L
(c) 1.5 x 10-4 mol / L
(d) 1.96 x 10-1 mol / L
Answer:
(c) 1.5 x 10-4 mol / L
Solution:
pH = 3.82 = – log10[H+]
∴ [H+] = 1.5 x 10-4 mol / litre
Question 54.
The pH of a solution at 25°C containing 0.10 M sodium acetate and 0.03 M acetic acid is …………..
(pKa for CH3COOH = 4.57)
(a) 4.09
(b) 5.09
(c) 6.10
(d) 7.09
Answer:
(b) 5.09
Solution:
pH = pKa + log \(\frac { [salt] }{ [acid] }\)
= 4.57 + log \(\frac { 0.10 }{ 0.03 }\) = 5.09
Question 55.
A weak acid is 0.1% ionised in 0.1 M solution. Its pH is …………..
(a) 2
(b) 3
(c) 4
(d) 1
Answer:
(c) 4
Solution:
For a monobasic acid [H+] = c.α
= \(\frac { 1 }{ 2 }\) x 0.001 = 10-4
pH = – log10[10-4] = 4
Question 56.
Which one of the following is not a buffer solution?
(a) 0.8 M H2S + 0.8 M KHS.
(b) 2 M C6H5NH2 + 2 M C6H5N
(c) 3 M H2CO3 + 3 M KHCO3
(d) 0.05 M KCIO4 + 0.05 M HCIO
Answer:
(d) 0.05 M KCIO4 + 0.05 M HCIO
Hint. HClO4 is a strong acid while buffer is a mixture of weak acid and its salt.
Question 57.
The pH of pure water or neutral solution at 50°C is …………… (pKw = 13.2613 at 50°C)
(a) 7.0
(b) 7.13
(c) 6.0
(d) 6.63
Answer:
(d) 6.63
Solution:
[H+] [OH–] = 10-13.26
[H+] = [OH–]
[H+] = \(\frac { { 10 }^{ \frac { -13.26 }{ 2 } } }{ 2 }\)
∴ pH = 6.63
Question 58.
Increasing order of acidic character would be ……………..
(a) CH3COOH < H2SO4 < HCO3
(b) CH3COOH < H2CO3 < H2SO
(c) H2CO3 < CH3COOH < H2SO4
(d) H2SO4 < H2CO3 < CH3COOH
Answer:
(d) H2SO4 < H2CO3 < CH3COOH
Question 59.
What is the pH of 1 M CH3COOH solution?. Ka of acetic acid is 1.8 x 10-5. K = 10-14 mol2 litre2.
(a) 9.4
(b) 4.8
(c) 3.6
(d) 2.4
Answer:
(a) 9.4
Solution:
CH3COO + H2O \(\rightleftharpoons\) CH3COOH + OH–
[OH–] = c x h

= 2.35 x 10-5
pOH = 4.62
pH + pOH = 14
pH = 14 – 4.62 = 9.38
Question 60.
4Na + O2 → 2Na2O
Na2O + H2O → 2NaOH
In the given reaction, the oxide of sodium is …………..
(a) Acidic
(b) Basic
(c) Amphoteric
(d) Neutral
Answer:
(b) Basic
Solution.
Na2O form NaOH so that it is basic oxide.
Question 61.
The pH of 0.001 M NaOH will be ………….
(a) 3
(b) 2
(c) 11
(d) 12
Answer:
(c) 11
Solution:
0.001 M NaOH means [OH–] 0.001 .
10-3 pOH = 3
pH + pOH = 14
pH = 14 – 3 = 11
Question 62.
The addition of pure solid sodium carbonate to pure water causes …………….
(a) an increase in hydronium ion concentration
(b) an increase in alkalinity
(c) No change in acidity
(d) A decrease in hydroxide ion
Answer:
(b) an increase in alkalinity
Hint.
Adding Na2CO3 to water makes the solution basic and hence pH increases from 7.
Question 63.
When solid potassium cyanide is added in water then ……………
(a) pH will increase
(b) pH will decrease
(c) pH will remain the same
(d) electrical conductivity will not change
Answer:
(a) pH will increase
Hint:
KCN + H2O \(\rightleftharpoons\) KOH + HCN.
KOH is a strong base and HCN is a weak acid. So pH will increase.
Question 64.
pH of a solution is 5. Its hydroxyl ion concentration is …………..
(a) 5
(b) 10
(c) 10-5
(d) 10-9
Answer:
(d) 10-9
Solution:
pH = 5 means [H+] = 10-5
pOH = 14 – pH = 14 – 5 = 9
[OH–] = 10-pOH = 10-9
Question 65.
Which one of the following is a buffer?
(a) CH3COOH + CH3COONa
(b) CH3COOH + CH3COONH4
(c) NaOH + NaCI
(d) CH3COOH + NH4CI
Answer:
(a) CH3COOH + CH3COONa
Question 66.
Which will have maximum pH?
(a) Distilled water
(b) 1 M NH3
(c) 1 M NaOH
(d) Water saturated by chlorine
Answer:
(c) 1 M NaOH
Hint:
NaOH has maximum [OH–] and minimum of [H+ ] and so maximum pH value.
Question 67.
pH of a solution is 9.5. The solution is …………..
(a) Neutral
(b) Acidic
(c) Basic
(d) Amphoteric
Answer:
(c) Basic
Solution:
If pH = 7 solution is neutral
pH < 7 solution is acidic
pH > 7 solution is basic
Question 68.
A solution has pH = 5, it is diluted 100 times, then it will become ……………….
(a) Neutral
(b) Basic
(c) unaffected
(d) more acidic
Answer:
(a) Neutral
Solution:
pH = 5 means [H+] = 10-5
After dilution [H+] = 10-5 / 100 = 10-7 M
[H+] from H2O cannot be neglected.
Total [H+] = 10-7 + 10-7 = 2 x 10-7
pH = 7 – 0.3010 = 6.6990 = 7
pH = 7 (Neutral)
Question 69.
By adding a strong acid to the buffer solution, the pH of the buffer solution ……………..
(a) remains constant
(b) increases
(c) decreases
(d) becomes zero
Answer:
(a) remains constant
Question 70.
pH of human blood is 7.4. Then H+ concentration will be ……………..
(a) 4 x 10-8
(b) 2 X 10-8
(c) 4 x 10-4
(d) 2 x 10-4
Answer:
(a) 4 x 10-8
pH = – log [H+]
7.4 = – log [H+]
7.4 = log 1 – log [H+]
log [H+] = log 1 – 7.4
log [H+] = 8.6
[H+] Antilog of 8.6
= 4 x 10-8
Question 71.
The highest pH 14 is given by ……………….
(a) 0.1 M H2SO4
(b) 0.1 M NaOH
(c) 1 N NaOH
(d) 1 N HCl
Answer:
(a) 0.1 M H2SO4
Solution:
[OH–] = 1
pOH = 0
pH + pOH = 14
pH = 14 – 0 = 14
Question 72.
Which of the following is not a Bronsted acid?
(a) CH3NH4
(b) CH3COO–
(c) H2O
(d) HSO4
Answer:
(b) CH3COO–
Hint:
Those substances which give a proton is called Bronsted acid, while CH3COO– doesn’t have a proton. So it is not a Bronsted acid.
Question 73.
Pure water is kept in a vessel and it remains exposed to atmospheric CO2 which is absorbed, then its pH will be
(a) greater than 7
(b) less than 7
(c) equal to 7
(d) depends on ionic production of water
Hint:
CO2 is acidic oxide which on dissolution in water develops acidic nature.
Question 74.
As the temperature increases, the pH of KOH solution ……………..
(a) will decrease
(b) will increase
(c) remains constant
(d) depends upon concentration of KOH solution
Answer:
(a) will decrease
Question 75.
The pH of millimolar HCl is ………….
(a) 1
(b) 3
(c) 2
(d) 4
Answer:
(b) 3
Solution:
pH = – log [H+]
[H+] = 10-3
pH = log 1 – log [H+]
= log 1 – log 10-3 = 3
Question 76.
The unit of ionic product of water K is ……………
(a) mol-1 L-1
(b) mol-2 L-2
(c) mol-2 L-1
(d) mol2 L-2
Answer:
(d) mol2 L-2
Question 77.
Review the equilibrium and choose the correct statement.
HClO4 + H2O \(\rightleftharpoons\) H3O+ + ClO4+
(a) HClO4 is the conjugate acid of H2O
(b) H3O is the conjugate base of H2O
(c) H2O is the conjugate acid of H3O
(d) ClO4 is the conjugate base of HCIO4
Answer:
(d) ClO4 is the conjugate base of HCIO4
Question 78.
Which of the following is the strongest conjugate base?
(a) CI–
(b) CH3COO
(c) SO42-
(d) NO2–
Hint:
CH3COO is a conjugate base of a weak acid.
CH3COOH \(\rightleftharpoons\) CH3COO + H+
Question 79.
Which one of the following substance has the highest proton affinity?
(a) H2O
(b) H2S
(c) NH3
(d) PH3
Answer:
(c) NH3
Question 80.
Which of the following is the strongest Lewis acid?
(a) BI3
(b) BBr3
(c) BCI3
(d) BF3
Hint:
Larger the size of the halogen atom less is the back donation of electrons into empty 2p orbital of B.
Question 81.
Which of the following is the weakest acid?
(a) HF
(b) HCI
(c) HBr
(d) HI
Answer:
(a) HF
Hint:
HF does not give proton easily.
Question 82.
Among the following, the weakest Lewis base is …………
(a) H–
(b) OH–
(c) CI–
(d) HClO3–
Hint:
CI– is a conjugate base of strong acid HCI.
Question 83.
Which of the following is not a Lewis acid?
(a) BF3
(b) AlCI3
(c) HCl
(d) LiAIH4
Hint:
It is a nucleophile and capable of donating electron pair and so it can act as Lewis base.
Question 84.
Which one of the following is called amphoteric solvent?
(a) Ammonium hydroxide
(b) Chloroform
(c) Benzene
(d) Water
Answer:
(d) Water
Hint:

Question 85.
Which of the following is non – electrolyte?
(a) NaCl
(b) CaCl2
(c) C12H22O11
(d) CH3COOH
Hint:
C12H22O11 is a sugar and non-electrolyte.
Question 86.
At infinite dilution, the percentage ionisation for both strong and weak electrolyte is ………….
(a) 1%
(b) 20%
(c) 50%
(d) 100%
Answer:
(d) 100%
Hint:
According to Ostwald’s dilution law, degree of ionisation is directly proportional to the dilution.
Question 87.
An acid HA ionises as HA \(\rightleftharpoons\) H+ + A– The pH of 1.0 M solution is 5. its dissociation constant would be …………..
(a) 1 x 10-5
(b) 1 x 10-10
(c) 5
(d) 5 x 108
Answer:
(b) 1 x 10-10
Question 88.
Three reactions involving H2PO4 are given below.
(i) H3PO4 + H2O → H3O+ + H2PO4–
(ii) H2PO4 + H2O → HPO42 + H3O+
(iii) H2PO4 + OH– → H3PO4 + O2–
In which of the above does H2PO4– act as an acid.
(a) (i) only
(b) (ii) only
(c) (i) & (iii)
(d) (iii) only
Answer:
(b) (ii) only
Question 89.
Which of the following is not a Lewis acid?
(a) CO
(b) SiCl4
(c) SO3
(d) Zn2+
Answer:
(c) SO3
CO does not contain vacant d-orbital.
Question 90.
A chemist dissolves an excess of BaSO4 in pure water at 25°C if its Ksp = 1 x 10-10 What is the concentration of Barium in the water?
(a) 10-14 M
(b) 10-5 M
(c) 10-15 M
(d) 10-6 M
Answer:
(d) 10-6 M
Question 91.
On addition of ammonium chloride to a solution of ammonium hydroxide ……………
(a) dissociation of NH4OH increases
(b) concentration of OH– increases
(c) concentration of OH– decreases
(d) concentration of NH4 and OH– increases
Hint:
Due to common ion effect.
Question 92.
The solubility product of a salt having a general formula MX2 in water is 4 x 10-2. The concentration of M2+ ions in the aqueous solution of the salt is ………………….
(a) 2.0 x 10-6 M
(b) 1.0 X 10-4 M
(c) 1.6 x 10-4 M
(d) 4.0 x 10-2 M
Answer:
(b) 1.0 X 10-4 M
Solution:
MX2 \(\rightleftharpoons\) M2++ 2X–
Ksp = (2s)2 (s) = 4s3

= 1.0 x 10-4M
Question 93.
The solubility of an aqueous solution of Mg(OH)2 be x then its Ksp is ……………
(a) 4 x3
(b) 108 x5
(c) 27 x4
(d) 9 x
Answer:
(a) 4 x3

Question 94.
What is the correct representation of the solubility product constant of Ag2CrO4?
(a) [Ag+]2 [CrO4-2]
(b) [Ag+] [CrO4-2]
(c) [2Ag+] [CrO4-2]
(d) [2Ag+]2 [CrO4-2]
Answer:
(a) [Ag+]2 [CrO4-2]
Ag2CrO4 \(\rightleftharpoons\) [2 Ag+] [CrO4-2]
Hence, Ksp = [Ag+]2 + [CrO4-2]
Question 95.
What is the pH value of \(\frac { N }{ 100 }\) KOH solution?
(a) 10
(b) 3
(c) 2
(d) 11
Answer:
(d) 11
Solution.
10-3 N KOH will give [OH–] = 10-3 M
pOH = 3
pH + pOH = 14
pH = 14 – 3 = 11
Question 96.
Which pair will show common ion effect?
(a) BaCI2 + Ba(NO3)2
(b) NaCI + HCI
(c) NH4OH + NH4CI
(d) AgCN + KCN
Answer:
(c) NH4OH + NH4CI
Question 97.
The sotubility of AgCI will be minimum in ………….
(a) 0.00 1 M AgNO3
(b) pure water
(c) 0.01 M CaCI2
(d) 0.01 M NaCl
Answer:
(c) 0.01 M CaCI2
Solution.
0.01 M CaCI2 gives maximum CI– ions to keep Ksp of AgCl constant, decrease in [Ag+] will be maximum.
Question 98.
Ionic product of water increases if ………….
(a) pressure is reduced
(b) H+ is added
(c) OH– is added
(d) temperature increases
Answer:
(d) temperature increases
Solution:
Kw increases with increase ¡n temperature.
Question 99.
pH of water is 7. When a substance Y is added in water, the pH becomes 13. The substance Y is a salt of …………..
(a) strong acid and strong base
(b) weak acid and weak base
(c) strong acid and weak base
(d) weak acid and strong base
Answer:
(d) weak acid and strong base
Question 100.
Sodium chloride is purified by passing HCl gas in a impure solution of sodium chloride. It is based on ………………
(a) Buffer action
(b) Common ion effect
(c) Association of salt
(d) Hydrolysis of salt
Answer:
(b) Common ion effect
II. Fill in the blanks.
- …………. theory does not explain the behaviour of acids and base in non aqueous solvents.
- According to Lowry Bronsted theory, an acid is defined as a substance that has a tendency to …………. a proton and base is a substance that has a tendency to …………. a proton.
- HCI and Cl are called …………. pairs.
- A …………. acid is a positive ion or an electron deficient molecule.
- …………. is an anion or neutral molecule that donates one lone pair of electrons.
- The ligands act as …………. and the central metal atoms that accepts a pair of electrons behave as a ………….
- Carbonium ion act as …………. and carbanion act as ………….
- Acids with …………. greater than ten are considered as strong acids and less than one are called weak acids.
- OH– and H2 are considered as ………….
- ClO4–, Cl–, HSO4, NO3– are considered as ………….
- …………. can act as an acid as well as base.
- At 25°C, the value of Kw is equal to ………….
- With the increase in temperature, Kw value is ………….
- The dissociation of water is an …………. reaction.
- Aqueous solution of HCl is …………. whereas aqueous solution of NH3 is ………….
- For neutral solutions, the concentration of [H3O+] as well as [OH–] is equal to at 25°C.
- The pH of battery acid is equal to ………….
- The pH of drain cleaner is equal to ………….
- …………. is the fraction of the total number of moles of a substance that dissociates at equilibrium.
- When the dilution increases by 100 times, the dissociation increases by ………….
- When dilution…………., the degree of dissociation of weak electrolyte also increases.
- The buffer present in the blood is ………….
- …………. introduced a quantity called buffer index ? as a quantitative measure of the
- When an acid reacts with a base, a salt and water are formed and the reaction is called ………….
- …………. is the conjugate base of the weak acid CH3COOH.
- Kidney stones are developed over a period of time due to the precipitation of ………….
- The pH of sea water is …………. than 7.
- O2- and H– are ………….
- All metal ions (or) atoms are ………….
- All anions are ………….
Answers:
- Arrhenius
- donate, accept
- Conjugate acid-base
- Lewis
- Lewis base
- Lewis base, Lewis acid
- Lewis acid, Lewis base
- Ka value
- very weak acids
- very weak base
- Water
- 1 x 10-14
- increases
- endothermic
- acidic, basic
- 1 x 10-17
- zero
- 14
- degree of dissociation ?
- 10 times
- increases
- H2CO3 and NaHCO3
- Vanslyke, buffer capacity
- Neutralization
- CH3COO–
- Calcium oxalate
- greater
- strong base
- Lewis acids
- Lewis bases
III. Match the following column – I & II using the correct code given below that.
Question 1.

Answer:
(a) 4 3 1 2
Question 2.

Answer:
(b) 3 1 4 2
Question 3.

Answer:
(c) 2 3 4 1
Question 4.

Answer:
(d) 4 3 2 1
Question 5.

Answer:
(a) 3 1 4 2
Question 6.
Answer:
(a) 2 3 4 1
Question 7.
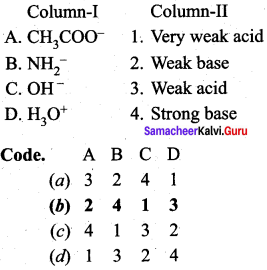
Answer:
(b) 2 4 1 3
Question 8.

Answer:
(c) 3 1 4 2
Question 9.
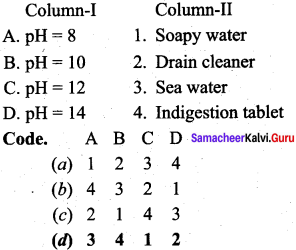
Answer:
(d) 3 4 1 2
Question 10.

Answer:
(a) 2 1 4 3
Question 11.
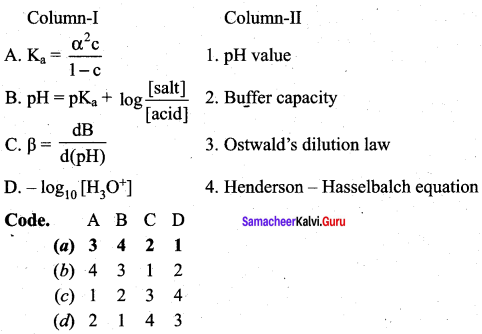
Answer:
(a) 3 4 2 1
IV. Assertion and reasons.
Question 1.
Assertion(A): In the process of dissolution of HCl in water, HCl act as acid and H2O act as base.
Reason (R): When HCl is dissolved in water, it donates a proton to water.
(a) Both A and R are correct and R explains A
(b) Both A and R are wrong
(c) A is correct but R is not the correct explanation of A.
(d) A is wrong but R is correct.
Answer:
(a) Both A and R are correct and R explains A
Question 2.
Assertion(A): When ammonia dissolved in water, H20 acts as an acid.
Reason (R): When ammonia is dissolved in water, it accepts a proton from water. According to Lowry – Bronsted theory, proton donor is acid and so water act as an acid.
(a) Both A and R are correct and R is the correct explanation of A
(b) Both A and R are wrong
(c) A is correct but R is not the correct explanation of A.
(d) A is wrong but R is correct. .
Answer:
(a) Both A and R are correct and R is the correct explanation of A
Question 3.
Assertion (A): In the reaction HCl + H2O \(\rightleftharpoons\) H3O+ + Cl–, HCl and Cl– are conjugate acid – base pair.
Reason (R): By Lowry – Bronsted theory, chemical species that differ only by a proton are called conjugate acid – base pair.
(a) Both A and R are correct and R is the correct explanation of A
(b) Both A and R are wrong
(c) A is correct but R is not the correct explanation of A
(d) A is wrong but R is correct.
Answer:
(a) Both A and R are correct and R is the correct explanation of A
Question 4.
Assertion(A): BF3 is a Lewis acid.
Reason (R): Boron has a vacant 2p orbital to accept the lone pair of electrons donated by any substance to form a new coordinate covalent bond.
(a) Both A and R are correct and R is the correct explanation of A
(b) Both A and R are wrong .
(c) A is correct but R is not the correct explanation of A.
(d) A is wrong but R is correct.
Answer:
(a) Both A and R are correct and R is the correct explanation of A
Question 5.
Assertion(A): in coordination compounds, the ligands acts as Lewis acid aid the central metal atom or ion act as Lewis base.
Reason (R): Ligands are capable of accepting of a pair of electrons donated by the central metal atom or ion.
(a) Both A and R are correct and R is the correct explanation of A
(b) Both A and R are wrong
(c) A is correct but R is not the correct explanation of A.
(d) A is wrong but R is correct.
Answer:
(b) Both A and R are wrong
Question 6.
Assertion(A): SiF4 can act as Lewis acid.
Reason (R): In SiF4, the central atom can expand its octet due to the availability of empty d – orbitais and can accept a pair of electrons.
(a) Both A and R are correct and R is the correct explanation of A
(b) Both A and R are wrong
(c) A is correct but R is wrong
(d) A is wrong but R is correct
Answer:
(a) Both A and R are correct and R is the correct explanation of A
Question 7.
Assertion(A): NH3, H2O, ROH all are examples of Lewis bases.
Reason (R): Molecules with one or more lone pairs of electrons act as Lewis bases.
(a) Both A and R are wrong
(b) A is correct and R is the correct explanation of A.
(c) A is wrong but R is correct.
(d) A is correct but R is wrong
Answer:
(b) A is correct and R is the correct explanation of A.
Question 8.
Assertlon(A): HCl is an strong acid while HCOOH is a weak acid.
Reason (R): HCI is completely ionised in water whereas HCOOH is paritally ionised in water.
(a) Both A and R are wrong
(b) A is wrong but R is correct
(c) A is correct but R is wrong
(d) Both A and R are correct and R is the correct explanation of A
Answer:
(d) Both A and R are correct and R is the correct explanation of A
Question 9.
Assertion(A): With the increase in temperature, the ionic product of water also increases.
Reason (R): The dissociation of water is an endothermic reaction.
(a) Both A and R are correct and R is the correct explanation of A
(b) A is correct but R is wrong
(c) A is wrong but R is correct
(d) Both A and R are wrong
Answer:
(a) Both A and R are correct and R is the correct explanation of A
Question 10.
Assertion(A): When dilution increases, the degree of dissociation of weak electrolyte also increases.
Reason (R): The degree of dissociation a is inversely proportional to concentration c. When the dilution increases by 100 times, the dissociation increases by 10 times.
(a) Both A and R are correct and R is the correct explanation of A
(b) Both A and R are wrong
(c) A is correct but R is wrong
(d) A is wrong but R is correct
Answer:
(a) Both A and R are correct and R is the correct explanation of A
Question 11.
Assertion(A): The addition of sodium acetate to acetic acid solution leads to the suppression in the dissociation of acetic acid.
Reason (R): This is due to common ion effect. i.e., CH3COOH and CH3COONa both contains CH3COO– ion as common.
(a) Both A and R are correct and R is the correct explanation of A
(b) BothA and R are wrong
(c) A is correct but R is wrong
(d) A is wrong but R is correct
Answer:
(a) Both A and R are correct and R is the correct explanation of A
Question 12.
Assertion(A): The solution of NH4CI has pH value less than 7.
Reason (R): The salt of weak base (NH4OH) and strong acid (HCl) is acidic in nature, when dissolved in water. So pH value is less than 7.
(a) Both A and R are correct and R is the correct explanation of A
(b) Both A and R are wrong
(c) A is correct but R is wrong
(d) A is wrong but R is correct
Answer:
(a) Both A and R are correct and R is the correct explanation of A
Question 13.
Assertion(A): pH = 7 signifies pure water.
Reason (R): pH = 7 means it is a neutral solution where [H3O+] [OH–]
(a) A is correct but R is wrong
(b) A is wrong but R is correct
(c) Both A and R are wrong
(d) A is correct and R does not explain A
Answer:
(b) A is wrong but R is correct
Question 14.
Assertion(A): A mixture of CH3COOH and CH3COONH4 is an acidic buffer.
Reason (R): An acidic buffer contains a weak acid and the salt of weak acid with strong base.
(a) A is correct but R is wrong.
(b) A is wrong but R is correct.
(c) Both A and R are correct and R is the correct explanation of A
(d) Both A and R are wrong
Answer:
(b) A is wrong but R is correct.
Question 15.
Assertion(A): Buffer mixture is the one whose pH remains constant even by addition of strong acid or strong base.
Reason (R): To resist changes in its pH on the addition of an acid or base, the buffer solution should contain both acidic as well as basic components so as to neutralise the effect of added acid or base.
(a) Both A and R are correct and R is the correct explanation of A
(b) Both A and R are wrong
(c) A is correct but R is wrong
(d) A is wrong but R is correct
Answer:
(a) Both A and R are correct and R is the correct explanation of A
V. Find the odd one out and give the reasons.
Question 1.
(a) HNO3
(b) Ba(OH)2
(c) H3PO4
(d) CH3COOH
Answer:
(b) Ba(OH)2
Reason: Ba(OH)2 is the base whereas the others are acids.
Question 2.
(a) NH3
(b) H2O
(c) RNH2
(d) BF3
Answer:
(d) BF3
Reason: BF3 is a Lewis acid whereas others are Lewis base.
Question 3.
(a) SiF4
(b) SF4
(c) FeCl3
(d) NH3
Answer:
(d) NH3
Reason: NH3 is a Lewis base whereas others are Lewis acid.
Question 4.
(a) HCl
(b) H2SO4
(c) CH3COOH
(d) HNO3
Answer:
(c) CH3COOH
Reason: CH3COOH is a weak acid whereas others are strong acids.
Question 5.
(a) HCOOH
(b) CH3COOH
(c) Lactic acid
(d) HCI
Answer:
(d)HCI
Reason: HCl is a strong acid whereas others are weak acids.
Question 6.
(a) HClO4
(b) HCI
(c) HSO4
(d) H2SO4
Answer:
(c) HSO4.
Reason: HSO4 is a very weak base whereas others are strong acid.
Question 7.
(a) NH2–
(b) O2–
(c) H–
(d) OH–
Answer:
(d) OH–
Reason: OH– is a very weak acid whereas others are strong bases.
Question 8.
(a) HNO2
(b) HF
(c) H2SO4
(d) CH3COOH
Answer:
(c) H2SO4
Reason: H2SO4 is a strong acid whereas others are weak acids.
Question 9.
(a) F–
(b) CH3COO
(c) O2
(d) NO2–
Answer:
(c) O2-
Reason: O2- is a strong base whereas others are weak bases.
Question 10.
(a) Vinegar
(b) Black coffee
(c) Sea water
(d) Orange juice
Answer:
(c) Sea water
Reason: Sea water is basic and has pH > 7 whereas others are acidic and have pH < 7.
Question 11.
(a) Baking soda
(b) Tomato
(c) Soapy water
(d) Drain cleaner
Answer:
(b) Tomato
Reason: Tomato has pH less than 7 and it is acidic whereas others have pH greater than 7 and they are basic.
Question 12.
(a) CH3COOH + CH3COONa
(b) NH4OH + NH4CI
(c) H2CO3 + NaHCO3
(d) NaOH + NaCl
Answer:
(d) NaOH + NaCl
Reason: NaOH + NaCl is not a buffer mixture whereas others are buffer mixtures.
VI. Find out the incorrect pair.
Question 1.
(a) HNO3, H2SO4
(b) Al(OH)3 , Mg (OH)2
(c) CH3COOH, HCOOH
(d) H2O, OH–
Answer:
(d) H2O, OH
Question 2.
(a) HCl , Cl–
(b) H2O, H3O+
(c) HNO3 , HNO2
(d) H2SO4, HSO4–
Answer:
(c) HNO3 , HNO2
Question 3.
(a) NH3, H2O
(b) ROH, ROR
(c) CN–, SCN–
(d) BF3, H2O
Answer:
(d) BF3, H2O
Question 4.
(a) BF3 , BF2
(b) Fe2, Fe3
(c) CaO, Mg(OH)2
(d) SiF4, SF4
Answer:
(c) CaO, Mg(OH)2
Question 5.
(a) Orange juice, Tomato juice
(b) Soapy water, Sea water
(c) Water, H3O
(d) Bleach , Ammonia solution
Answer:
(c) Water, H3O
VII. Find out the correct pair.
Question 1.
(a) HNO3, Ba(OH)2
(b) CH3COOH , HCI
(c) H3O+, Cl–
(d) HCl + H2SO4
Answer:
(d) HCl + H2SO4.
Question 2.
(a) BF3, NH4+
(b) CH2– , CH3+
(c) Fe2+, Fe3+
(d) (CH3)3C+ , CH2 = CH2
Answer:
(c) Fe2+, Fe3+
Question 3.
(a) H3O+, HCI
(b) HSO4, NO2
(c) HNO2, H2
(d) HCl, Cl–
Answer:
(d) HCI, CI–
Question 4.
(a) Orange, Black coffee
(b) Baking soda,, Water
(c) Ammonia, Stomach acid
(d) Bleach , Tomato
Answer:
(a) Orange , Black coffee
Question 5.
(a) NH4OH + NaOH
(b) NaOH + NaCl
(c) CH3COOH + CH3COONa
(d) CH3COOH + CH3COONH4
Answer:
(c) CH3COOH + CH3COONa
VIII. Answer the following.
Question 1.
What are the general characteristics of acid and base?
Answer:
- Acid tastes sour, turns the blue litmus to red and reacts with metals such as zinc and produces hydrogen gas.
- Base tastes bitter, turns the red litmus to blue and soapy to touch.
Question 2.
Explain the Arrhenius concept of acid and base with example.
Answer:
1. According to Arrhenius concept, an acid is a substance that dissociates to give hydrogen ions in water. For example,

2. Similarly a base is a substance that dissociates to give hydroxyl ions in water. For example,

Question 3.
What are the limitations of Arrhenius concept?
Answer:
1. Arrhenius theory does not explain the behaviour of acids and base in non-aqueous solvents such as acetone, tetrahydro furan.
2. This theory does not account for the basicity of the substances like ammonia which do not possess hydroxyl group.
Question 4.
What is meant by strong acid and weak acid? Explain with example.
Answer:
1. A strong acid is the one that is almost completely dissociated in water.
HCl + H2O → H3O+ + Cl–
2. A weak acid is the one that is partially dissociated in water.
CH3COOH +H2O \(\rightleftharpoons\) H3O + CH3COO–
Question 5.
Give two examples for
- Strong acid
- Strong base
Answer:
- Strong acid: HCIO4, H2SO4
- Strong base: NH2–, O2-
Question 6.
Give two examples for
- Very weak acid
- Very weak base
Answer:
- Very weak acid: OH–,H2
- Very weak base: Cl–, ClO4–
Question 7.
Give two examples for
- Weak acid
- Weak base
Answer:
- Weak acid: HF, CH3COOH
- Weak base: F, CH3COO
Question 8.
What is meant by auto ionisation of water?
Answer:
Pure water has a little tendency to dissociate. i:e., one water molecule donates a proton to another water molecule. This is known as auto ionisation of water.

Question 9.
Define – ionic product of water.
Answer:
H2O + H2O \(\rightleftharpoons\) H3O + OH
The dissociation constant for the above ionisation is given as,
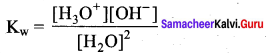
The concentration of pure water is one.
i.e., [H2O]2 = 1
K = [H3O+] [OH–]
Kw = ionic product of water.
Kw = 1 x 10-14 at 25°C
Question 10.
Kw = 1 x 10-14 at 25°C. Justify this statement.
Answer:
1. Experimentally found that the concentration of H3O in pure water is 1 x 10-7 at 25°C.
2. Since the dissociation of water produces equal number of H3O4 and OH–, the concentration of OH– is also equal to 1 x 10– at 25°C. The ionic product of water at 25°C is
Kw = [H3O+] [OH–]
= [1 x 10-7] [1 x 10-7]
Kw = [1 x 10-14]
Question 11.
With increase in temperature, Kw also increases. Why?
Answer:
- All equilibrium constant Kw. is also a constant at a particular temperature.
- The dissociation of water is an endothermic reaction.
- With the increase in temperature, the concentration of H3O+ and OH– also increases and hence the ionic product also increases.
Question 12.
Aqueous HCl is an acidic solution whereas aqueous NH3 is a basic solution. Justify this statement.
Answer:
HCI + H2O \(\rightleftharpoons\) H3O+ + Cl– in this case, in addition to auto ionisation of water, HCI molecule also produces H3O ion by donating a proton to water and hence [H3OJ> [OH]. It means that the aqueous HCI solution is acidic. Similarly in basic solution such as aqueous NH3, [OH–] > [H3O+] and it is basic.
Question 13.
What is the statement of Ostwald’s dilution law.
Answer:
When dilution increases, the degree of dissociation of weak electrolyte also increases.
![]()
This statement is known as Ostwald’s dilution law.
Question 14.
Define – Salt hydrolysis.
Answer:
Salts completely dissociate in aqueous solution to give their constituent ions. The ions so produced are hydrated in water. ¡n certain cases, the cation, anion ?r both react with water and the reaction is called salt hydrolysis. e.g.,
NaOH(aq) + HNO3(aq) → NaNO3(aq) + H2O(1)
Question 15.
What is meant by conjugate acid-base pair? Find the conjugate acid / base for the following species
Answer:
HNO2, CH–, HCIO4, OH–, CO32-, S2-
An acid-base pair which differs by a proton only (HA \(\rightleftharpoons\) A– + H+) is known as conjugate acid-base pair.
Conjugate acid: HCN, H2O, HCO3–, HS–.
Conjugate base: NO2–, ClO4–, O2–.
Question 16.
Which of the following are Lewis Acids?
H2O, BF3, H+ and NH4+
Answer:
BF3, H+ ions are Lewis acids.
Question 17.
What will be the conjugate bases for the Bronsted acids? HF, H2SO4 and H2CO3?
Answer:
Conjugate bases: F–, HSO4–, HCO3–.
Question 18.
Write the conjugate acids for the following Bronsted bases:
NH2–, NH3 and HCOO–
Answer:
NH3, NH4 and HCOOH
Question 19.
The species H2O, HCO3–, HSO4– and NH3 can act both as Bronsted acid and base. For each case, give the corresponding conjugate acid and base.
Answer:
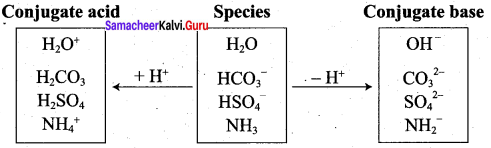
Question 20.
Classify the following species into Lewis acids and Lewis bases and show how these can act as Lewis acid I Lewis base?
(a) OH– ions
(b) F–
(c) H+
(d) BCI3
Answer:
(a) OH– ions can donate an electron pair and act as Lewis base.
(b) F– ions can donate an electron pair and act as Lewis base.
(a) H+ ions can accept an electron pair and act as Lewis base.
(b) BCl3 can accept an electron pair since Boron atom is electron deficient. It is a Lewis acid.
Question 21.
Predict the acidic, basic or neutral nature of the solutions of the following salts:
NaCI, KBr, NaCN, NH4NO3, NaNO2, KF.
Answer:
NaCN, NaNO2, KF solutions are basic, as they are salts of strong base, weak acid.
NaCl, KBr solutions are neutral, as they are salts, of strong acid, strong base.
NH4NO3 solution is acidic, as it is a salt of strong acid, weak base.
Question 22.
Ionic product of water at 310 K is 2.7 x 10-14 What Is the pH of neutral water at this temperature?
Answer:
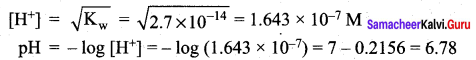
Question 23.
The aqueous solution of sugar does not conduct electricity whereas when sodium chloride is added to water, it conducts electricity. Justify this statement.
Answer:
1. Sugar is a non electrolyte and when it dissolves in water, there will be no ionisation takes place. If there is no free ions, it does not conduct electricity.
2. When sodium chloride is added to water, it is completely ionised to give Na ions and Cl– ions. Due to the presence of ions, they will be possibility of electhcal conductance. Because ions are carriers of electric current.
Question 24.
A reaction betwen ammonia and boron trifluoride is given below
![]()
Identify the acid and base in the reaction. Which theory explain it?
Answer:
![]()
1. In the above reaction BF3 is an acid and NH3 is the base.
2. Lewis concept explain it as follows

3. A Lewis acid is an electron deficient molecule and capable of accepting a pair of electrons and a Lewis base is electron rich molecule and capable of donating a pair of electrons.
Question 25.
The salt of strong acid and strong base does not undergo hydrolysis. Explain.
Answer:
1. In this case, neither the cations nor the anions undergo hydrolysis. Therefore the solution remains neutral.
2. For example, in the aqueous solution of NaCl, its ions Na+ and Cl– ions have no tendency to react with H+ or OH– ions of water.
This is because the possible products of such reaction are NaOH and HCI which are completely dissociated. As a result, there is no change in the concentration of W and OH– ions and hence the solution continues to remain neutral.
Samacheer Kalvi 12th Chemistry Ionic Equilibrium 3 Mark Questions ans Answers
Question 1.
Explain Lowry – Bronsted theory of acid and base.
Answer:
1. According to Lowry-Bronsted theory, an acid is defined as a substance that has a tendency to donate a proton to another substance and base is a substance that has a tendency to accept a proton from other substance.
2. An acid is a proton donor and a base is a proton acceptor.
3. When HCI is dissolved in H2O, HCI donates a proton to H2O. Thus HCI behaves as an acid and H2O is a base.
HCI + H2O \(\rightleftharpoons\) H3O+ + Cl–
Question 2.
Explain the reaction of water with ammonia by proton theory.
Answer:
1. When ammonia dissolved in water, it accepts a proton from water. In this case, ammonia (NH3) acts as a base and H2O is acid.
2. The reaction is represented as
![]()
3. The species that remains after the donation of a proton is a base (Base1) and is called the conjugate base of Bronsted acid (Acid1). In other words, chemical species that differ only by a proton are called conjugate acid base pairs Conjugate acid – base pair

Question 3.
Explain about the strength of acids on the basis of Ka value.
Answer:
1. Ka is called the ionisation constant or dissociation constant of the acid. It measures the strength of an acid.
2. Acids such as HCI, HNO3 are almost completely? onised and hence they have high Ka value i.e., Ka for HCI at 25°C is 2 x 106.
3. Acids such as formic acid and acetic acid are partially ionised in solution and have low Ka value. i.e., Ka for acetic acid 1.8 x 10-5 at 25°C
4. Acids with Ka value greater than ten are considered as strong acids and less than one considered as weak acids.
Question 4.
Write 3 formulas of strong acids, strong bases and weak acids.
Answer:
- HClO4, HCI, H2SO4 – are strong acids
- NH2–, O2-, H– – are strong bases
- HNO2, HF, CH3COOH are weak acids
Question 5.
pH of a neutral solution is equal to 7. Prove it.
Answer:
1. in neutral solutions, the concentration of [H3O+] as well as [OH–] are equal to 1 x 10-7M at 25°C.
2. The pH of a neutral solution can be calculated by substituting this [H3O+] çoncentration in the expression
pH = – log10 [H3O+]
= – log10 [1 x 10-7]
= – ( – 7)log \(\frac { 1 }{ 2 }\) = + 7 (l) = 7
3. pH = 7 for a neutral solution
Question 6.
Derive the relation between pH and pOH
Answer:
pH = – log10 [H3O] ………………(1)
pOH = – log10 [OH–] ……………(2)
Adding equations (1) and (2),
pH + pOH = (- log10 [H3O+) + ( – log10[OH–])
= – [(log10[H3O]) + (log10 [OH–)]
pH + pOH = – log10[H3O+] [OH–]
[H3O] [OH–] = Kw
pH + pOH = – log Kw
pH + pOH = pKw
[pKw = – 1og10Kw]
At 25°C, the ionic product of water Kw = 1 x 10-14.
pKw = – 1og1010-14 = 14 log1010 = 14
pKw = 14
pH + pOH = 14 at 25°C.
Question 7.
When the dilution increases by 100 times, the dissociation increases by 10 times. Justify this statement.
Answer:
(i). Let us consideran acid with Ka value 4 x 104. We are calculating the degree of dissociation of that acid at two different concentration 1 x 10-2 M and 1 x 10-4 M using Ostwalds dilution law
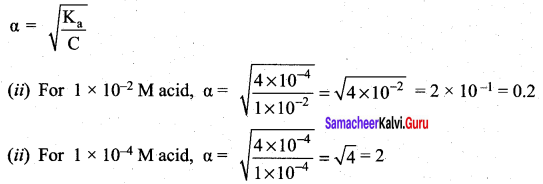
(iv) i.e., when the dilution increases by 100 times (concentration decreases from 1 x 10-2M to 1 x 10-4M), the dissociation increases by 10 times.
(v) When dilution increases, the degree of dissociation of weak electrolyte also increase. (Ostwalsd’s dilution law).
Question 8.
What Is buffer solution? Give an example for an acidic buffer and a basic buffer.
Answer:
- Buffer is a solution which consists of a mixture of weak acid and its conjugate base (or) a weak base and its conjugate acid.
- This buffer solution resists drastic changes in its pH upon addition of a small quantities of acids (or) bases and this ability is called buffer action.
- Acidic buffer solution, Solution containing acetic acid and sodium acetate. Basic buffer solution, Solution containing NH4O and NH4Cl.
Question 9.
Define buffer capacity and buffer index.
Answer:
- The buffering ability of a solution can be measured in terms of buffer capacity.
- Buffer index ?, as a quantitative measure of the buffer capacity.
- It is defined as the number of gram equivalents of acid or base added to 1 litre of the buffer solution to change its pH by unity.
- β = \(\frac { dB }{ d(pH) }\). dB = number of gram equivalents of acid / base added to one litre of buffer solution. d(pH) = The change in the pH after the addition of acid / base.
Question 10.
How is solubility product is used to decide the precipitation of ions?
Answer:
1. When the product of molar concentration of the constituent ions i.e., ionic product exceeds the solubility product then the compound gets precipitated.
2. When the
ionic Product > Ksp precipitation will occur and the solution is super saturated. ionic Product < Ksp no precipitation and the solution is unsaturated. ionic Product = Ksp equilibrium exist and the solution ¡s saturated.
3. By this way, the solubility product finds useful to decide whether an ionic compound gets precipitated when solution that contain the constituent ions are mixed.
Question 11.
Derive the value of solubility product from molar solubility.
Answer:
1. Solubility can be calculated from molar solubility.i.e., the maximum number of moles of the solute that can be dissolved in one litre of the
solution.
2. For a solute XmYn
Xm Yn(s) \(\rightleftharpoons\) mXn+(aq) + n Ym-(aq)
3. From the above stoichiometrically balanced equation, it is clear that I mole of Xm Yn(s) dissociated to furnish ‘m’ moles of x and ‘n’ moles of Y. If’s’ is the molar solubility of Xm Ynthen
Answer:
[Xn+] = ms and [Ym-] = ns
Ksp = [Xn+]m [Ym-]n
Ksp = (ms)m (ns)n
Ksp = (m)m (n)n (s)m+n
Question 12.
The concentration of hydrogen ions ¡n a sample of soft drink is 3.8 x 10-3m. What is the. pH value? Whether the soft drink is acidic (or) basic?
Answer:
pH = – log10 [H3O+]
= – log10 [3.8 x 10–]
= – log 3.8 + 3
= 3 – 0.5798 = 2.4202
pH = 2.42
When pH < 7, the soft drink is acidic.
Question 13.
The pH of a sample of vinegar is 3.76. Calculate the concentration of hydrogen ion in it.
Answer:
pH = – log10 [H3O+]
= – log10 = – pH = – 3.76
= \(\overline{4}\).24
[H3O+] = antilog \(\overline{4}\).24
= l.738 x 10-4
[H3O+] = 1.74.x 10-4M
Question 14.
The ionisation constant of HF, HCOOH, HCN at 298 K are 6.8 x 10-4, 1.8 x 10-4 and 4.8 x 10-9 respectively. Calculate the ionisation constant of the corresponding conjugate base.
Answer:
1. HF, conjugate base is F
Kb = Kw/Ka = \(\frac{1 \times 10^{-4}}{6.8 \times 10^{-4}}\) = l.47 x 10-11 = l.5 x 10-11
2. for HCOO–
Kb = \(\frac{1 \times 10^{-14}}{1.8 \times 10^{-4}}\) = 5.6 x 10-11
3. for CN–
Kb = \(\frac{1 \times 10^{-14}}{4.8 \times 10^{-4}}\) = 2.8 x 10 -6
Question 15.
The pH of 0.1 M solution of cyanic acid (HCNO) is 2.34. Calculate the ionization constant of the acid and its degree of ionization in the solution.
HCNO \(\rightleftharpoons\) H+ + CNO–
pH = 2.34 means – log [H+] = 2.34 or log [H+] = – 2.34 = 3.86
or
[H+] = Antilog 3.86 = 4.57 x 10-3 M
[CNO–] = [H+] = 4.57 x 10-3 M

Question 16.
The Ionization constant of nitrous acid is 4.5 x 10-4. Calculate the pH of 0.04 M sodium nitrite solution and also its degree of hydrolysis.
Answer:
Sodium mtrite is a salt of weak acid, strong base. Hence,
Kh = 2.22 x 10-11 Kw/Kb = 10-14/(4.5x 10-4)

[OH–] = ch = 0.04 x 2.36 x 10-5 = 944 x 10-7
pOH = – log (9.44 x 10-7) = 7 – 0.9750 = 6.03
pH = 14 – pOH = 14 – 6.03 = 7.97
Question 17.
What is the minimum volume of water required to dissolve 1 g of calcium sulphate at 298K. For calcium sulphate, Ksp = 9.1 x 10-6.
Answer:
CaSO4(s) Ca2(aq) + SO2-4(aq)
If ‘s’ is the solubility of CaSO4 in moles L–, then Ksp = [Ca2+] x [SO42-] = s2
or
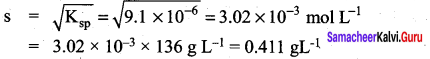
= 3.02 x 10-3 x 136gL-1 = 0.411 gL-1
(Molar mass of CaSO4 = 136 g mol-1)
Thus, for dissolving 0.441 g, water required = I L
For dissolving 1g, water required = \(\frac { 1 }{ 0.411 }\)L = 2.43L
Question 18.
- Point out the differences between ionic product and solubility product.
- The solubllity of AgCI in water at 298 K is 1.06 x 10-5 mole per litre. Calculate is solubility product at this temperature.
Answer:
1. Ionic product
- It is applicable to all types of solutions.
- Its value changes with the change in con centration of the ions.
Solubility product
- It is applicable to the saturated solutions.
- It has a definite value for an electrolyte at a constant temperature.
2. The solubility equilibrium in the saturated solution is
AgCl (s) \(\rightleftharpoons\) Ag+(aq) + Cl– (aq)
The solubility of AgCl is 1.06 x 10-5 mole per litre.
[Ag+(aq)] = 1.06 x 10-5 mol L-1
[Cl– (aq)] = 1.06 x 10-5 mol L-1
Ksp = [Ag+(aq)] [Cl– (aq)]
= (1.06 x 10-5 mol L-1) x (1.06 x 10-5 mol L-1)
= 1.12 x 10-2 moI2 L-2
Question 19.
The value of K of two sparingly soluble salts Ni(OH)2 and AgCN are 2.0 x 10-15 and 6 x 10-17 respectively. Which salt is more soluble? Explain.
Answer:

Ni(OH)2 is more soluble than AgCN.
Question 20.
If 0.561 g KOH is dossolved in water to give 200 mL of solution at 298 K, calculate the concentration of potassium, hydrogen and hydroxyl ions. What is its pH?
Answer:

Samacheer Kalvi 12th Chemistry Ionic Equilibrium 5 Marks Questions and Answers
Question 1.
Differentiate Lewis acids and Lewis bases.
Answer:
Lewis Acids
- Lewis acids are substances that can accept one or more lone pair of electrons.
- All metal ions (or) atoms can act as Lewis acids. Examples: Fe2+, Fe3+, Cu2+, Cr3+
- Molecules that contain a polar double bond can act as Lewis acids. Examples: SO2, CO2, SO3
- Molecules in which the central atom can expand its act due to the availability of empty d-orbitais can act as Lewis acid. Example: SiF4, SF4, FeCI3
- Carbonium ion (CH3)3C+ can act as Lewis acid
- Electron deficient molecules such as BF3, AlCl3, BeF2 act as Lewis acid (electron pair acceptors)
Lewis Bases
- Lewis bases are substances that can donate one or more lone pair of electrons.
- All anions can act as Lewis bases. Examples: F–, Cl–, CN–, SO42-
- Molecules that contain carbon-carbon multiple bond. Example: CH2 = CH2, CH = CH
- All metal oxides can act as Lewis bases. Examples : CaO, MgO, Na2O
- CH2– carbanion cari act as Lewis acid
- Electron rich molecules such as NH3, H2O, ROH, R – O – R, R – NH2 act as Lewis base (Electron pair donors)
Question 2.
Explain about the ionisation of weak acid and how K2 is derived?
Answer:
1. Weak acids are partially dissociated ¡n water and there is an equilibrium between the undissociated acid and its dissociated ions.
2. Consider the ionisation of weak monobasic acid HA in water
HA + H2O \(\rightleftharpoons\) H3O+ + A– …………….(1)
3. Applying law of chemical equilibrium, the equilibrium constant Kc is given by the expression

4. In dilute solutions, water is present in large excess, hence its concentration may be taken as constant say K. Further H3O+ indicates hydrated hydrogen ions, for simplicity, it may be replaced by H+. So the equation (2) becomes

5. The product of two constants K and K gives another constant. Let is be K2

The constant Ka is called dissociation constant of weak acid.
Question 3.
Explain Buffer action with suitable example.
Answer:
Buffer action:
1. Let us consider buffer solution containing CH3COOH and CH3COO Na. The dissociation the buffer components occur as below.

2. If an acid is added to this mixture, it will be consumed by the conjugate base CH3COO– to form undissociated weak acid. i.e., the increase in the concentration of H+ does not reduce the pH significantly.
CH3COO–(aq) + H+(aq) → CH3COOH(aq)
3. If a base is added, it will be neutralised by H3O and the acetic acid is dissociated to maintain the equilibrium. Hence the pH is not altered.

4. On the addition of an acid (or) base to a buffer solution, there will be no change in its pH value. Because the buffer solution should contain both acidic as well as basic components so as to neutralise the effect of added acid (or) base at the same time, these components should not consume each other.
Question 4.
Prove the buffer action of acetic acid and sodium acetate by the addition of 0.01 mol of solid sodium hydroxide.
Answer:
1. Consider one litre of buffer solution containing 0.8 m CH3COOH and O.8m CH3COONa. Assume that the volume change due to the addition of 0.01 mol of solid NaOH is negligible. Ka for CH3COOH is l.8 x 10-5.
2.

3. The dissociation constant for CH3COOH is given by

The above expression shows that the concentration of H+ is directly proportional to

degree of dissociation of CH3COOH = α
4.

5. Given that Ka for CH3COOH is 1.8 x 10-5
[H+] = 1.8 x 10-5
pH = – log [H+]
= – log [ 1.8 x 10-5]
= 5 – log 1.8
= 5 – 0.26
pH = 4.74
6. After adding 0.01 moI NaOH to I litre of buffer. Given that volume change due to the addition of NaOH is negligible. [OH–] = 0.01 M. The consumption of OH– are expressed by the following equation.
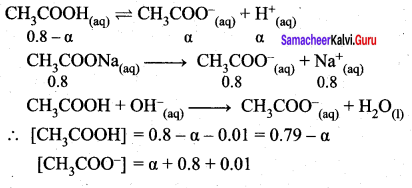

7. The addition of a strong base (0.01 M NaOH) increased the pH only slightly i.e., from 4.74 to 4.75. So the buffer action is verified.
Question 5.
DerIve Henderson – Hasselbalch equation
Answer:
1. The concentration of hydronium ion in acidic buffer solution depends on the ratio of concentration of the weak acid to the concentration of its conjugate base present in the solution. i.e.,

2. The weak acid is dissociated only to a small extent. Moreover due to common ion effect, the dissociation is further suppressed and hence the equilibrium concentration of the acid is nearly equal to the initial concentration of the unionised acid. Similarly the concentration of the conjugate base is nearly equal to the initial concentration of the added salt.

3. [Acid] and [Salt] represent the initial concentration of the acid and salt, respectively used to prepare the buffer solution.
4. Taking logarithm on both sides of the equation
![]()
5. reverse the sign on both sides
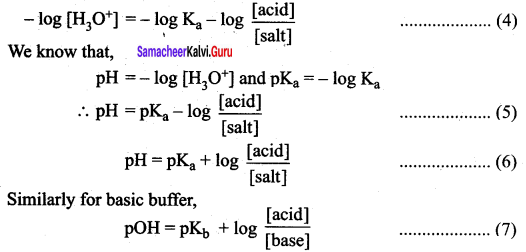
Eqaution (6) & (7) are called Henderson – Hasselbalch equations.
Question 6.
Explain about the hydrolysis of salt of strong acid and a strong base with a suitable example.
Answer:
1. Let us consider the neutralisation reaction between NaOH and HNO3 to give NaNO3 and water.
NaOH(aq) + HNO3(aq) → NaNO3(aq) + H2O(1)
2. The salt NaNO3 completely dissociates in water to produce Na+ and NO3 ions
![]()
3. Water dissociates to a small extent as
H2O(1) H+(aq) + OH–(aq)
Since [H+] = [OH–], water is neutral.
4. NO3 ion is the conjugate base of strong acid HNO3 and hence it has no tendency to react withH+,
5. Similarly Na is the conjugate acid of the strong base NaOH and it has no tendency to react with OH
6. It means that there is no hydrolysis. In such cases [H+] (OH–), pH is maintained and there fore the solution is neutral.
Question 7.
Explain about the hydrolysis of salt of strong base and weak acid. Derive the value of Kh for that reaction.
Answer:
1. Let us consider the reaction between sodium hydroxide and acetic acid to give sodium acetate and water.
NaOH(aq) + CH3COOH(aq) \(\rightleftharpoons\) CH3COONa(aq) + H2O(1)
2. In aqueous solution, CH3COONa is completely dissociated as follows.
CH3COONa(aq) CH3COO–(aq) + Na+(aq)
3. CH3COO is a conjugate base of the weak acid CH3COOH and it has a tendency to react with H+ from water to produce unionised acid. But there is no such tendency for Na+ to react with OH–
4. CH3COO–(aq) + H2O(1) CH3COOH(aq) + OH–3 and therefore [OH–] > [H+], in such cases, the solution is basic due to the hydrolysis and pH is greater than 7.
5. Relationship between equilibrium constant, hydrolysis constant and the dissociation constant of acid is derived as follows:
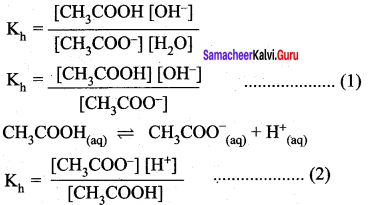
Equation (1) x (2)
Kh.Ka = [H+] [OH–]
[H+] [OH–] = Kw
Kh.Ka = Kw
Kh value in terms of degree of hydrolysis (h) and the concentration of salt (c) for the equilibrium can be obtained as in the case of Ostwald’s dilution law Kh = h2C and [OH–] =![]()
Question 8.
DerIve the value of pH of salt solution in terms of Ka and concentration of electrolyte.
Answer:
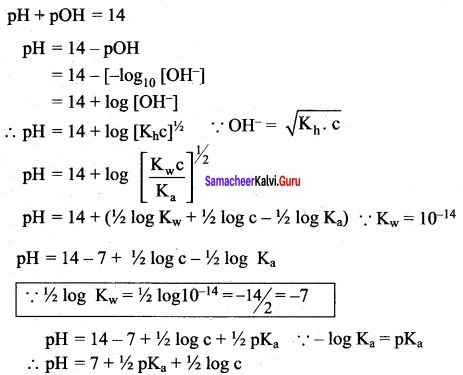
Question 9.
Explain about the hydrolysis of salt of strong acid and weak base. Derive Kh and pH for that solution.
Answer:
1. Consider a reaction between strong acid HCl and a weak base NH4OH to produce a salt NH4CI and water
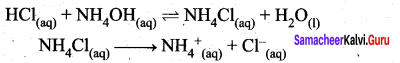
2. NH4 is a strong conjugate acid of the weak base NH4OH and it has a tendency to react with OH- from water to produce unionised NH4 as below,
![]()
3. There is no such tendency shown by Cl– and therefore [H+] > [OH–] the solution is acidic and the pH is less than 7.
4. In the salt hydrolysis of strong base and weak acid, we have to derive a relationship between Kh and Kb as
Kh . Kb = Kw
5.
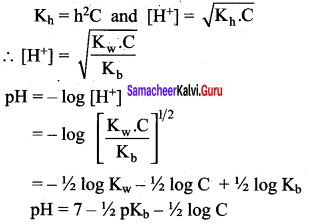
Question 10.
Discuss about the hydrolysis of salt of weak acid and weak base and derive pH value for the solution.
Answer:
1. Consider the hydrolysis of ammonium acetate
CH3COONH4(aq) → CH3COO–(aq) + NH+4(aq)
2. In this case both the cation (NH4+) and (CH3COO–) anion have the tendency to react with water.
CH3COO– + H2O \(\rightleftharpoons\) CH3COOH + OH–
NH4+ + H2O \(\rightleftharpoons\) NH4OH + H3
3. The nature of the solution depends on the strength of acid (or) base i.e., if Ka > Kb, then the solution is acidic and pH < 7, if Ka < Kb then the solution is basic and pH > 7. If Ka = Kb, then the solution is neutral.
4. The relation between the dissociation constant Ka, Kb and hydrolysis constant is given by the following expression.
Ka . Kb. Kh = Kw
5. pH of the solution
pH = 7 + \(\frac { 1 }{ 2 }\)pKa – \(\frac { 1 }{ 2 }\)pKb
Question 11.
It has been found that the pH of a 0.01 M solution of an organic acid Is 4.15. Calculate the concentration of the anion, the Ionization constant of the acid and its pKa.
Answer:
HA \(\rightleftharpoons\) H+
pH = log [H+] or log [H+] = – 4.15 = 5.85
[H+] = 7.08 x 10-5 M = 7.08 x 10-5 M
[A–] = [H+] = 7.08 x 10-5M
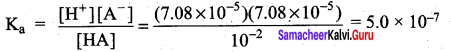
pKa = – logKa = – log (5.0 x 10-7) = 7 – 0.699 = 6.301
Question 12.
Assuming complete dissociation, calculate the pH of the following solutions.
(i) 0.003 M HCl
(ii) 0.005 M NaOH
(iii) 0.002 M HBr
(iv) 0.002 M KOH
Answer:
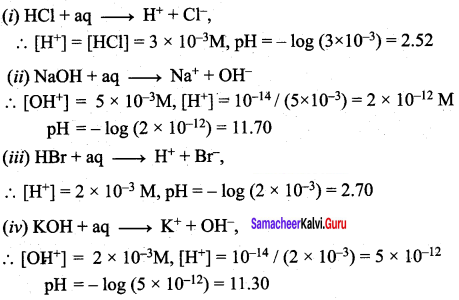
Question 13.
What ¡s the pH of 0.001 M aniline solution? The ionisation constant of aniline is 4.27 x 10-10. Calculate degree of ionization of aniline in the solution. Also calculate the ionisation constant of the conjugate acid of anile.
Answer:
1. C6H5NH2 + H2O \(\rightleftharpoons\) C6H5NH3 + OH–
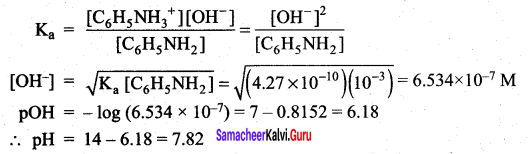
2. Also

3. pKa + pKb = 14 (for a pair of conjugate acid and base)
pKb = – log (4.27 x 10-10) = 10 – 0.62 = 9.38
pKa = 14 – 9.38 = 4.62
i.e., – log Ka 4.62 or log Ka = – 4.62 =
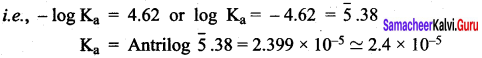
Question 14.
Calculate the degree of ionization of 0.05 M acetic acid If its pKa value is 4.74. How is the degree of dissociation affected when its solution also contains
- 0.01 M
- 0.1 M HCI
Answer:
PKa = i.e., – log Ka = 4.74
or log Ka = 4 . 74 = 5.26
Ka = 1.82 x 10-5
![]()
In presence of HCI, due to high concentration of H+ ion, dissociation equilibrium will shift backward, Le., dissociation of acetic acid will decrease.
1. In presence of 0.01 M HCI, if x is the amount dissociated, then

2. In the presence of 0.1 M HCl, if y is the amount of acetic acid dissociated, then at equilibrium


Question 15.
The ionization constant of acetic acid is 1.74 x 10-5. Calculate the degree of dissociation of acetic acid in its 0.05 M solution. Calculate the concentration of acetate ions in the solution and its pH.
Answer:

Common Errors
- Acid and Base – Definiton
- pH value
- Buffer mixture
- Conjugate Acid base pair
Rectifications

- pH neutral
- pH less than 7 – Acid
- pH more than 7 – Base
- Always either weak acid and its salt (or) weak base and its salt.
- They differ by H+. For e.g., CH3COOH. Its conjugate base is CH3COO–. H2O – Acid and its conjugate base is OH–
Hope you love the Samacheer Kalvi 12th Chemistry Chapter Wise Material. Clearly understand the deep concept of Chemistry learning with the help of Tamilnadu State Board 12th Chemistry Chapter 8 Ionic Equilibrium Questions and AnswersPDF. Refer your friends to and bookmark our website for instant updates. Also, keep in touch with us using the comment section.